>40 yo → greatest infertility.
• Fecundity: Probability that a single menstrual cycle results in live birth
History (Fertil Steril 2004;82:S169)
• Gravidity, parity, Preg outcomes/assoc complications
• Age at menarche, cycle length & characteristics, dysmenorrheal moliminal sx
• Methods of contraception used in the past; frequency & timing of intercourse
• Duration of infertility & results of any prev eval & rx
• H/o thyroid dz, pelvic or abdominal pain, galactorrhea, hirsutism, dyspareunia
• Full medical & surgical Hx, including STIs & PID, prior abd/pelvic Surg
• Prev abn pap smears & any subseq rx
• Current meds including supplements & allergies
• Social history (SHx). Occupation, tobacco, EtOH, drug use
• Family history (FHx) of birth defects, mental retardation, or infertility
• Partner’s reproductive Hx (conceptions/children in other pairings, testicular trauma, chronic medical conditions, & meds). Remember male factor on diff dx.
Physical Examination
• Weight & BMI. Thyroid enlargement, nodule, or tenderness. Breast exam. Signs of androgen excess or acanthosis nigricans. Pelvic or abdominal tenderness, masses. Vaginal or cervical abnormality, secretions, or discharge. Uterine size, shape, position, & mobility. Adnexal mass or tenderness. Cul-de-sac mass, tenderness, nodularity.
Diagnostic Evaluation
• Ovulatory fxn: Oligomenorrhea (>35 d btw menses) or amenorrhea (>3 mo btw menses) → no further w/u. Luteal phase (cycle day 21 or 7 d after ovulation) serum prog >6 ng/mL confirms ovulation. Urinary LH (commercial ovulation predictor kits) generally reliable & correlate w/ serum LH. Serum FSH/LH ratio & estradiol (cycle day 3) or AMH (any time in cycle) indicate ovarian reserve. If ovulatory dysfxn → TSH, prolactin, & FSH for etiology.
• Anatomy assessment: HSG evaluates tubal patency & uterine cavity, endometrial polyps, submucosal fibroids. Schedule 2–5 d after last menses. Rx doxycycline 100 mg PO BID for 5 d if h/o PID or dilated tubes (Obstet Gynecol 2009;113(5):1180). Beware HSG contrast can → tubal spasm (false + tubal blockage). TVUS shows uterine cavity contours & small intrauterine lesions. Sonohysterography (saline infusion sonogram) more accurate than HSG, as accurate as hysteroscopy for cavity assessment. 2D & 3D TVUS more sensitive than HSG for fibroids & polyps. Hysteroscopy for definitive dx + rx of cavity pathology. Laparoscopy definitive for tubal & pelvic pathology. Chromopertubation (the injection of indigo carmine dye through cervical canula w/ direct intra-abdominal observation of tubal spill for eval of tubal occlusion) & rx of mild dz (fimbrial agglutination, adhesion, endometriosis).
• See also male factor w/u & other diagnoses, below
PREMATURE OVARIAN INSUFFICIENCY (POI)
Definition & Epidemiology (Obstet Gynecol 2009;113:1355; Lancet 2010;376:911)
• Decline in nml ovarian fxn in a woman <40 yo. A form of hypergonadotropic hypogonadism. 0.3% of reproductive age  ; 5–10%
; 5–10%  w/ secondary amenorrhea.
w/ secondary amenorrhea.
Etiology
• Accelerated follicular atresia due to genetic syn (Turner XO → oocyte apoptosis; fragile X premutation → oocyte toxic prot). Autoimmune ovarian failure secondary to systemic autoimmune dz (check for type 1 DM, thyroiditis, hypoadrenalism). Ovarian toxins (chemo w/ alkylating agents, XRT, smoking, infxn such as mumps or CMV).
• Abn follicular stimulation due to defects in steroidogenic enzymes or defects in ovarian gonadotropin receptors (eg, FSH receptor mut)
• Result is ↓ ovarian estrogen production → ↓ negative feedback on pituitary → ↑ FSH, LH
Clinical Manifestations
• Primary or secondary infertility. Irreg menses vs. primary or secondary amenorrhea
• W/ fragile X – mental retardation, ataxia, premature ovarian failure
• W/ Turner syn – short stature, shield chest, web neck, low hairline, low set ears, aortic coarct, streak ovaries
• ↓ estrogen w/ primary infertility → impaired secondary sexual dev, dyspareunia (secondary to vaginal dryness), decreased bone density
• ↓ estrogen & secondary infertility → hot flashes, night sweats, emotional lability, dyspareunia, decreased bone density
Initial Workup
• ↓ estrogen → ↑ FSH, ↑ LH. POI if:
FSH >10 mIU/mL (except during the midcycle preovulatory LH surge)
FSH > LH w/ E2 <50 pg/mL (× 2 if ↑ FSH) = absent/nonfunctioning follicles
• Clomiphene citrate challenge test – check FSH on cycle day 3 & 10 after 100-mg clomiphene PO daily on cycle day 5–9; ↑ FSH after clomid sugg low ovarian reserve
• AMH–secretion by small preantral & early antral follicle granulosa cells reflects size of primordial follicle pool, declines w/ age, undetectable at menopause. Early marker of ovarian reserve, & AMH level is not cycle dependent. AMH >1 ng/mL – adequate ovarian reserve.
• AFC by transvaginal US – high variability, useful if equivocal labs
Follow-up Studies
• Genetics. Karyotype (identify individuals w/ any form of gonadal dysgenesis characterized by an absent or abn X chromo & those w/ any portion of a Y chromo), genetic testing for FMR1 gene permutations
• Adrenal autoantibodies by immunofluorescence assay
• Anti-islet cell Ab (given association w/ type 1 DM)
• Serum TSH, thyroid-stimulating Ig, thyroid peroxidase antibodies
• Bone mineral density to detect osteopenia
Treatment and Medications
• HT to ↓ sx of estrogen deficiency & prevent bone loss
• Daily calcium (1200–1500 mg) + Vit D (600–800 IU) for bone health
• Exogenous androgen – unclear role in mgmt; no high-quality evid
• Clinician sensitivity, additional psychological support
• IVF using donor oocytes – controversial in women w/ Turner syn
POLYCYSTIC OVARIAN SYNDROME (PCOS)
Definition (Nat Rev Endocrinol 2011;74:219)
• A d/o of ovarian fxn characterized by anovulation, elevated androgen levels, & polycystic ovaries. A/w obesity & insulin resistance (metabolic syn). Different diagnostic criteria used:
1990 NIH – NICHD – hyperandrogenism or hyperandrogenemia, oligoanovulation, & exclusion of other endocrine disorders
2003 Rotterdam criteria – 2 of following 3: Clinical or biochemical hyperandrogenism, oligo- or anovulation, polycystic ovaries. Other endocrine disorders must be excluded.
2006 androgen excess – PCOS society – clinical or biochemical hyperandrogenism w/ oligo-/anovulation &/or polycystic ovaries
Epidemiology & Pathophysiology
• 6–10% of women, depending on diagnostic criteria. Uncertain etiology, but hyperandrogenism may cause ovulatory dysfxn & abn gonadotropin secretion.
• Androgen excess → follicular arrest & ↑ LH. Hyperinsulinemia may also → follicular arrest & phenotypic features.
• Presentation may include excess body or facial hair, frequent shaving/plucking, irreg menstruation, infertility, alopecia, acne, obesity, metabolic syn.
Physical Exam
• Assess weight & BMI, hair pattern/growth, thyroid, galactorrhea (prolactin-secreting tumor), acanthosis nigricans
• Deep voice, male pattern facial/body hair, clitoromegaly may suggest androgen-secreting tumor or congen adrenal hyperplasia
Diagnostic Workup
• Document oligo- or anovulation by Hx, serum progesterone, or urinary LH testing
• Labs: Consider testing for serum androgens esp if no clinical hyperandrogenism – or – if frank virilization. TSH, FSH, & prolactin if pt anovulatory. 75 g, 2-h oral gluc tol test for women w/ hyperandrogenism w/ anovulation + acanthosis nigricans +
Obesity (BMI > 30 kg/m2, or >25 in Asian pop) + FHx of T2DM or GDM (Fertil Steril 2012;97(1):28)
• TVUS ovaries: ≥12 follicles in each ovary measuring 2–9 mm in diameter, &/or ovarian volume >10 mL indicates polycystic ovaries
• Endometrial bx if long Hx of oligomenorrhea due to ↑ endometrial cancer
Treatment (Fertil Steril 2008;89:505)
• Exercise & weight loss improve ovulation rate – 1st-line rx
• In women not attempting Preg, low-dose combination OCP may ↓ hyperandrogenism & risk of endometrial cancer
• Clomiphene citrate 1st-line ovulation induction in women desiring Preg (see below)
• Limit to 6 ovulatory cycles before considering 2nd-line rx
• Ovulation induction w/ exogenous gonadotropins is 2nd-line therapy. IVF is 3rd-line therapy.
TUBAL FACTOR INFERTILITY
Definition & Epidemiology (Curr Opin Infect Dis. 2004;17(1):49;2005)
• Infertility caused by obliteration of the fallopian tube, usually by prior pelvic infxn. 20–30% of infertility may be tubal factor. Very common.
Etiology
• Obliteration of the fallopian tube or damage to fimbriae by infectious or inflamm process. Most cases caused by prev Hx of PID.
• Less common causes are inflammation related to endometriosis, inflamm bowel dz, & surgical adhesions
Clinical Manifestations
• Usually asymptomatic but may have dysmenorrhea & dyspareunia if endometriosis
• Hx of PID, ectopic Preg, or prior pelvic Surg
Diagnostic Workup/Studies
• HSG – diagnostic, but also may ↑ fertility
• Consider laparoscopy w/ chromopertubation if endometriosis suspected
• Chlamydia Ab testing may be helpful to screen pts at high risk for tubal factor infertility, but role of testing has not been clearly defined yet (Fertil Steril 1994;62:305)
Treatment and Medications (Fertil Steril 2012;97:539)
• Prox tubal obst → tubal cannulation
• Mild hydrosalpinges → laparoscopic fimbrioplasty or neosalpingostomy
• Irreparable hydrosalpinges → IVF. Salpingectomy or prox tubal occlusion improves IVF Preg rates.
• Decision to pursue Surg vs. IVF based on age of woman, number of children desired, extent of tubal dz
RECURRENT PREGNANCY LOSS (RPL)
Definition (N Engl J Med 2010;363:1740)
• 3 or more consecutive Preg losses before 20 w gest; some recommend w/u after 2 consecutive losses, esp if age >35 yo or pt requests
Epidemiology & Etiology
• 1% of all couples attempting Preg. Increases in women <18 yo & >35 yo.
• Most very early (<10 w) miscarriages due to aneuploidy
• Autoimmune dz, anatomic abnormalities, & thrombophilias may lead to vascular insufficiency for developing conceptus, leading to miscarriage
Evaluation
• Determine actual gestational age at time of miscarriage rather than time of onset of sx if poss
• Ask about Hx of thrombosis or prev fetal death; Hx of prev Preg w/ breech presentation, dysmenorrhea, or menorrhagia (may suggest uterine anomaly or fibroids); chronic medical conditions such as thyroid dz, diabetes, or autoimmune dz such as lupus; smoking, obesity, EtOH use, caffeine use
Diagnostic Workup (Int J Gynaecol Obstet 2002;78:179)
• Parental karyotype for balanced translocations. Aneuploid karyotype of prior loss fetuses makes other causes less likely.
• Antiphospholipid Ab syn w/u: Lupus anticoagulant (RVVT and hexagonal phospholipid, or aPTT with mixing studies), β2 glycoprotein Ab (IgM/IgG), anticardiolipin Ab (IgM/IgG). Need 2 positive tests 12 w apart to make dx.
• Consider thrombophilia w/u only if pt has a Hx of thromboembolism. Test for Factor V Leiden, prothrombin G20210A mut, prot C, prot S, antithrombin III deficiency.
• Evaluate uterine cavity using HSG, hysteroscopy, sonohysterography, or transvaginal US
• No dx is made in 50% of cases of recurrent Preg loss (Fertil Steril 2012)
Treatment (N Engl J Med 2010;363:1740)
• If positive antiphospholipid antibodies, heparin 5000 U subcut twice daily & low-dose ASA can ↓ miscarriage rates. Low-molecular-weight-heparin dose not established.
• If genetic abnormality such as balanced translocation present, up to 70% live birth w/o intervention, but may consider preimplantation genetic screening
• If uterine septum → hysteroscopic resxn. Repair of bicornuate or unicornuate uterus not necessary as obstetric outcome often good & repair has higher risk.
• No rx for women w/ thrombophilias thus far has been found beneficial
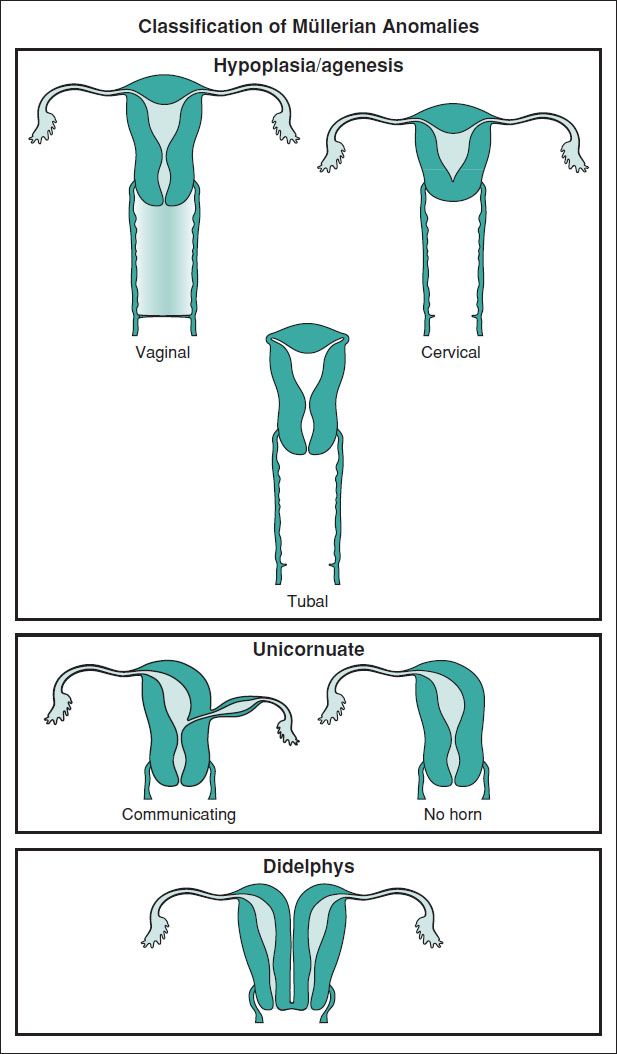
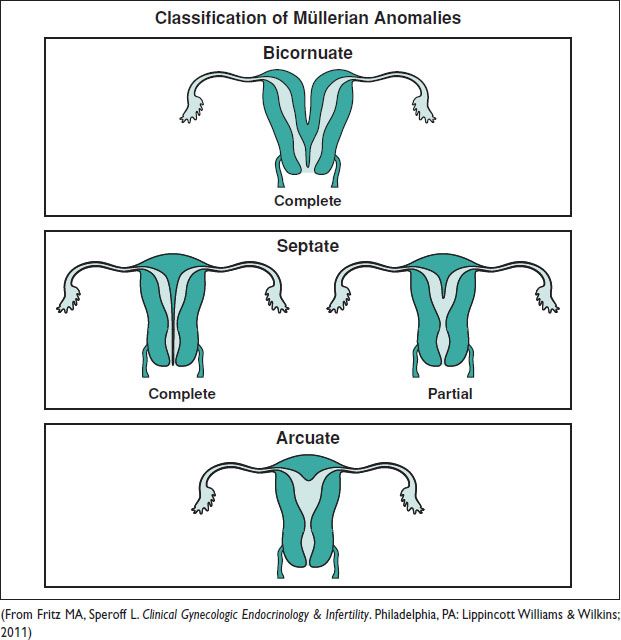
MÜLLERIAN ANOMALIES
Definitions and Epidemiology (Hum Reprod Update 2011;17:761)
• An anomaly of the uterus, tubes, or upper vagina due to failure of dev, fusion, or resorption of Müllerian structures
5–6% of women (arcuate uterus 3.9%, septate uterus 2.3%, bicornuate 0.4%, unicornuate 0.3%, didelphys 0.3%). ↑ to 8% w/ infertility. ↑ to 13% w/ recurrent miscarriage. ↑ to 25% w/ mixed infertility & recurrent miscarriage. Many also have a GU abnormality.
Etiology (Fritz MA, Speroff L. Clinical Gynecologic Endocrinology & Infertility. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.)
• Sporadic: Multifactorial & polygenic. 46 XX (92%); sex chromo mosaicism (8%).
• Risk factors: Hypoxia during Preg, MTX, DES, thalidomide, radiation, viral infxn
• Vertical fusion failure (canalization) → urogenital sinus & Müllerian tubercle separate
• Lateral fusion failure (duplication) → failure to merge bilateral Müllerian ducts
• Dev of the uterus, fallopian tubes, & upper vagina:
2 Müllerian (paramesonephric) ducts form from celomic epithelium beside the wolffian (mesonephric) ducts. In the absence of the SRY gene on the Y chromo & subseq MIS or AMH, Müllerian ducts proliferate & grow caudally & medially extending from the vaginal plate of the urogenital sinus to beside the developing ovary. In absence of testosterone, wolffian ducts involute. Canalization of the ducts occurs w/ a cranial lumen opening into peritoneal cavity. The paired ducts fuse in the midline forming the body of the uterus & the unfused lateral arms form the fallopian tubes. Resorption of medial aspects.
• Dev of urogenital sinus forms lower vagina, bladder, urethra
Urogenital sinus develops from the ventral portion of the cloaca (terminal hindgut; confluence of the urethra, rectum, & vagina). The caudal aspect of the paramesonephric ducts fuses w/ the urogenital sinus to form the vaginal & cervix.
Figure 8.1 Types of congenital uterine anomalies