Microbial colonies accumulate in hand crevices and on tips of fingers, illustrated in darkened areas here.
A patient had three routine ICSI procedures carried out using frozen–thawed samples of testicular sperm. On each occasion, oocytes were fertilized, but all of the embryos lysed within a few days. The frozen specimen was sent for microbial culture, with a result that showed the presence of gram-negative bacilli, identified as Morganella morganii; the bacilli were probably being injected into oocytes along with the sperm. This case also highlights the need to prepare frozen–thawed samples via repeated washing through media containing antibiotics, even when the sperm is of poor quality or obtained from surgical sperm retrieval.
Anything brought into the lab can be a source of contamination, including consumables, equipment (new or repaired), fresh gametes (e.g. specimen containers for semen samples), frozen gametes, or embryos imported from another lab: this includes infections that may be carried on the outside of dry shippers. Staged entry to the embryology lab is therefore preferable to a single entrance leading to joint embryology and andrology labs.
The wide range of problems associated with microbial contamination in an IVF setting has been comprehensively reviewed (Pomeroy, 2010). An online survey that included 32 experienced laboratory directors reported the incidence and type of contamination found in their IVF laboratories; the results revealed that semen (32%) and inadequate sterile technique (23%) were the most common sources of microbial contamination. The contaminant was identified as bacteria in 49% of the cases, and as yeast or mould (fungi) in 51%. The majority occurred in culture systems that included antibiotics in the media, and therefore the microbes identified (E. coli, Aspergillus, C. albicans, gram-negative cocci) had probably acquired resistance to the antibiotics being used. The most common sources of contamination in the cases documented were semen, the technician, and oil.
Normal flora
The human body acts as a natural host to a micro-ecosystem of flora that normally do no harm, unless the natural balance of their habitat is disturbed. Surface tissues in contact with the environment are always colonized with a mixture of microbes, known as “normal flora”, and this mixture varies according to site, age, sex, diet, health, and temperature of the environment. The results of a semen or vaginal culture will usually report organisms that are incidental findings of normal flora; antibiotic treatment may disturb the balance, allowing overgrowth of more insidious and resistant pathogens. Normal flora that are benign to the patient can be carried over into an IVF culture system: unlike the human body, embryos have no “defense” mechanism, other than the physical barrier of the zona pellucida, and are highly vulnerable to infection – most cases of overt bacterial contamination will result in non-viable embryos.
Skin is colonized with a variety of normal flora, and shedding skin contributes heavily to the bacterial load of the operating theater and laboratory environment (Dalstrom et al., 2008; Pomeroy, 2010). A poor standard of hygiene, cleaning, and waste disposal will result in a much higher risk of transmitting infection to and from patients, staff, and incubators/embryos. An untidy cluttered lab also increases the risk of contamination; simple cleaning can reduce the environmental microbial load by 80%, and the use of a disinfectant can reduce it to 95% (Pomeroy, 2010). Lab protocols must incorporate a discipline of rigorous hygiene, with
thorough hand-washing
use of sterile gloves where appropriate
regular cleaning of walls, floors, and installations
autoclaving lab clothing and autoclavable instruments.
Other precautions include HEPA air filtration under positive pressure to ensure that clean air flows into the laboratory, as well as the use of antibiotics in culture media. Table 9.2 shows the active spectrum and mode of action of antibiotics commonly used in culture media. Penicillin has a half-life of 2 hours at 37 °C, and therefore this has now been generally replaced with a stable form of gentamicin. Antibiotic stability is readily affected by temperature, pH, and light, and media must therefore be handled appropriately with this in mind: repeated removal from the refrigerator/opening of bottles and holding media at temperatures >4 °C affect stability of the antibiotics. The following guidelines are recommended:
1. Keep containers tightly closed and refrigerated at all times.
2. After use, discard bottles of culture media that have been warmed.
3. Use media within a few days after opening.
4. Use smaller volume (single-use) bottles whenever possible.
Commercial media are supplied in bottles with a range of different volumes, from 20 ml to 60 ml; the volumes appropriate for individual laboratories will depend upon the daily caseload.
| Antibiotic | Effective against | Mode of action |
|---|---|---|
| Penicillin | Gram-positive bacteria | Disrupts cell wall stability |
| Streptomycin | Gram-positive bacteria | Disrupts protein synthesis |
| Gentamicin | Gram-positive bacteria Gram-negative bacteria | Disrupts protein synthesis |
Semen samples
Semen samples are not sterile, even when produced under conditions of strict hygiene. A wide variety of bacteria can be isolated from semen, and the species vary with different country populations. Common normal flora include Staphylococcus epidermidis, streptococci (non-hemolytic, ß-, and Streptococcus viridians), diptheroids, coliforms, etc. Ureaplasma and mycoplasma species can also be isolated from semen samples (Cottell et al., 1997). More sensitive methods that use PCR to detect bacterial DNA (Kiessling et al., 2008) revealed that 65% of the men tested were found to carry bacteria, including gram-positive anaerobic cocci, Corynebacterium spp., Lactobacilli and Streptococci. A similar study indicated that many of the bacteria in semen are also present in the vaginal flora of some women, suggesting that heterosexual partners may share bacteria (Hou et al., 2013).
Semen may also be contaminated with Chlamydia trachomatis, a sexually transmitted infection whose incidence continues to rise each year: 89 million new cases were thought to occur internationally in 2010 (Akande et al., 2010). C. trachomatis can bind to sperm and form inclusion bodies (Wolner-Hanssen & Mardh, 1984); its concentration is reduced, but not necessarily removed, during sperm preparation procedures (Al-Mously et al., 2009). Routine clinical diagnosis of chlamydial infection is made by testing a urine specimen or urethral swab, but the organism can be present in semen even if these are negative. Eley & Pacey (2011) reported the results from 11 studies, which showed chlamydial detection in both semen and urine; there was a 20% detection rate in urine only and a 23% detection rate in semen only (Eley & Pacey, 2011).
Mycoplasma species are frequently found in semen, and this microorganism can contaminate a tissue culture without being readily detectable. Ultrastructurally, mycoplasma microorganisms appear as moderately electron-dense spherical particles that adhere to cell membranes and affect the axonemal complex of the sperm mid-piece sections (Sylla et al., 2005). Effects of “silent” mycoplasma contamination include growth inhibition, pH shifts, substrate depletion, induction of oxidative stress, ammonia production, secretion of harmful metabolites, and effects on nucleic acid and protein synthesis with preferential depletion of certain amino acids (Smith, 1960; Macpherson, 1966).
Follicular fluid
Follicular fluid (FF) is not sterile, and some of the micro-organisms identified in FF have been associated with potentially adverse effects on ART treatment (Pelzer et al., 2013). A prospective study assessed the presence of micro-organisms in samples from 262 women: vaginal swabs were taken after vaginal prep by washing, in parallel with FF harvested at the time of oocyte retrieval. All of the vaginal swabs cultured showed evidence of 1–11 different species, with Lactobacillus spp., Bifidobacterium spp., and Staphylococcus spp. the most prevalent. Bacteria were also cultured from 99% of the FFs tested; 71% of the FFs contained bacteria that were also present in vaginal swabs taken from the same patient, and were thus considered to be contaminated. Of the FFs, 29% were colonized with bacteria that were not cultured in the vaginal swab from that patient. Bacteria cultured from FF included Lactobacilli, Actinomyces spp., Propionibacteria, Staphyloccoccus spp., Peptostreptococcus spp., Bifidobacterium spp., Streptococcus agalactae, E. coli, and Enterococcus faecalis. Propionibacterium spp., Streptococcus spp., Actinomyces spp., Staphylococcus spp., and Bifidobacterium spp. appeared to be opportunistic pathogens and were related to adverse IVF outcomes. In a separate prospective study, 100% of FF tested showed the presence of micro-organisms after culture (Cottell et al., 1996). Interestingly, the presence of lactobacilli seemed to correlate with positive outcomes, perhaps because lactobacilli produce hydrogen peroxide and lactate, which inhibit the growth of other microbes. A further study in which catheter tips were cultured after ET confirmed that the growth of lactobacilli from the vagina and from the ET catheter was associated with an improved pregnancy rate; the live birth rate was reduced in cases associated with the recovery of Streptococcus viridans from the ET catheter (Moore et al., 2000).
Fungal contamination
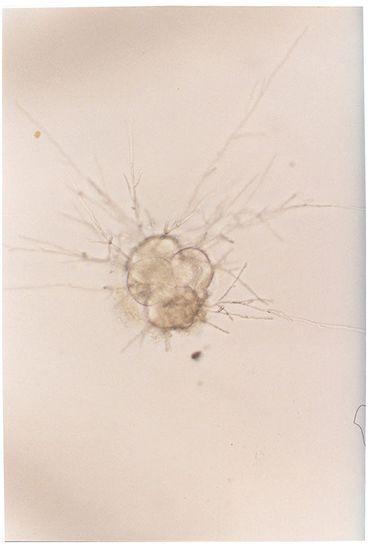
Yeast infections (Candida spp., Fig. 9.2) can be introduced into an IVF culture either from patients during follicular aspiration, or from the skin of laboratory or clinical staff. Fungi such as Aspergillus (Fig. 9.3) are frequent airborne contaminants, and may sometimes be traced to a heating or air conditioning system. Culture medium does not contain anti-fungal agents. Fungal infection is known to compromise embryo development, although it can be removed from the culture by repeated washing (Ben-Cherit et al., 1996). Embryos that have been exposed to fungal infection within a culture dish can still potentially develop into viable pregnancies, provided that the embryo is immediately and effectively removed from infected medium. Patients must be informed and give their consent before this can be undertaken.
Human embryos with yeast (C. albicans) contamination in the culture dish.
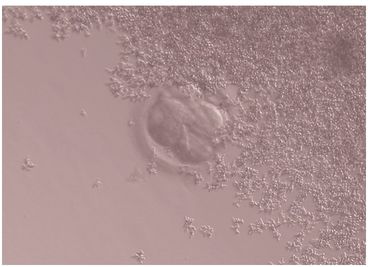
Embryo with spherical budding cells (blastoconidia) in the medium.
Embryo surrounded by filamentous budding elongated cells (pseudohyphae).

IVF culture dish contaminated with Aspergillus.
A female patient had been treated for tuberculosis 2 years prior to ART treatment; her partner tested negative for TB. On Day 2 post-OCR, bacteria were identified surrounding the embryos, even though cultures on Days 0 and 1 showed no bacterial growth. The embryos subsequently arrested development. Upon further investigation, it was discovered that the patient had developed a chronic persistent infection with a resistant TB strain, due to previous antibiotic treatment. The conclusion reached was that the bacteria had been transmitted via the egg collection procedure.
A couple were treated in the UK for routine IVF treatment. On the day of sample production, eggs from the parasite Schistosoma haematobium were identified in a fresh semen sample; the female partner was free of infection. Questioning the couple directly revealed that the man had been walking barefoot in a Malawi lake during a trip earlier in the year. He had acquired the parasite from the waters – Schistosoma travels subcutaneously to reside in the bladder/urethra and would have been expelled during ejaculation. All oocytes were vitrified for future insemination, and the patient was prescribed anti-parasitic medication. Subsequent samples were shown to be parasite-free and ICSI treatment was continued on the warmed oocytes (Shearer et al., 2013) (Fig. 9.4).
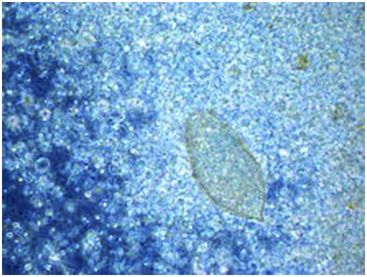
Schistosoma haematobium observed in a wet slide preparation during a diagnostic semen analysis (image taken at 400x).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


