21.4.2 Workload
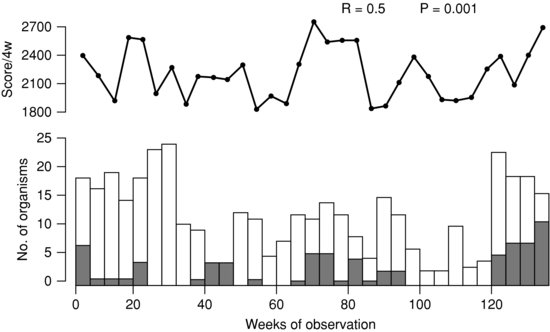
The transmission of gentamicin-resistant Gram-negative bacilli on a UK NICU did not correlate with antibiotic use but correlated with two indicators of workload (Figure 21.2).25 Transmission correlated with the number of unit baby days each week and even better with a score which also took into account the level of nursing care.25
Figure 21.2 Significant association between workload score (top line) and transmission of antibiotic-resistant Gram-negative bacilli on a neonatal unit. Reprinted with permission from Reference 25.
One problem with assessing workload is that sicker infants require intensive nursing. NICUs with high-risk infants need more nursing staff and a higher nurse:patient ratio than NICUs looking after low-risk infants. However, less mature, high-risk infants are at increased risk of nosocomial infections. A large prospective UK study looked at outcomes in relation to patient volume, staffing and workload in 186 NICUs and attempted to adjust the outcomes for risk. The incidence of nosocomial bacteraemia increased with increasing patient volume.26
Two studies examined the effect of nursing workload on mortality but not specifically on nosocomial infections.27,28An Australian single-centre study found improved infant survival with the highest infant/staff ratio.27 A UK study of 54 NICUs found risk-adjusted mortality was inversely related to having specialist neonatal nurses (OR 0.67; 95% CI 0.42–0.97). Increasing the ratio to 1:1 of nurses with neonatal qualifications to high-risk infants was associated with a 48% decrease in risk-adjusted mortality (OR: 0.52, 95% CI 0.33, 0.83).28 These studies emphasize the need for adequate staffing with specialist neonatal nurses. Hand washing by nurses29 and physicians30 deteriorates with increased workload. This largely explains the increased incidence of nosocomial infections with increasing workload, and shows adequate staffing of neonatal units is critical to prevent nosocomial infections.
21.5 Outbreak management
What constitutes an outbreak? Logically an outbreak should refer to more than one related case of infection on a neonatal unit. Clinically, however, an ‘outbreak’ might refer to a situation requiring an immediate enhanced infection control response. On occasion, a single infant developing sepsis (or even colonization) with a virulent organism, for example Pseudomonas (Section 21.5.1) or pertussis (Section 21.9), might suggest the need for action to prevent an outbreak. Similarly, diagnosing TB or varicella in a parent or staff member requires immediate action. In contrast, in some situations (e.g. a cluster of necrotizing enterocolitis in high-risk infants with no common organism identified and no known precipitating factors) a vigorous infection control response is likely to increase workload without benefit.
A systematic review31 used the Outbreak Database, a free worldwide database for nosocomial outbreaks (available on www.outbreak-database.com), to compare neonatal nosocomial infection outbreaks with those occurring in other intensive care units. The authors reviewed 276 outbreaks from NICUs and 453 from other ICUs. Enterobacteriaceae were responsible for NICU outbreaks significantly more often than in other ICUs, where non-fermenters predominated. On average, 23.9 patients and 1.8 health-care workers were involved in NICU outbreaks. The average mortality in NICU outbreaks was 6.4% (1.5 newborns on average). In 48.6% of NICU outbreaks the authors were unable to identify the source. The most important infection control measures were significantly more often implemented in NICUs than in other ICUs. Currently there are 328 neonatal outbreaks on the database, which is a useful resource for research.31
In a systematic review of 125 articles regarding outbreaks and sporadic incidents of health-care-associated infections, Gram-positive cocci, viruses and fungi predominated in reports from Western units, while Gram-negative enteric bacilli, non-fermenters and fungi predominated in resource-poor settings.32 Most outbreaks in either Western or developing countries were attributed to poor infection control practices.32
Table 21.1 outlines a suggested approach to outbreaks.33,34 A common question is whether or not to investigate for an environmental source. Outbreaks caused by water-loving organisms are more likely to have an environmental source and the possibility of a common environmental source should be considered earlier in outbreaks due to non-fermentative and other Gram-negative bacilli. Common modes of spread are outlined in Table 21.2.
Table 21.1 Guidelines for management of an outbreak.
1. Decide whether or not an outbreak or the potential for an outbreak exists 2. If yes, convene meeting to decide: a. is there likely to be a common source b. where to nurse index case(s) c. how to protect uninfected infants (and staff) d. how to detect new cases, for example stool cultures for gram-negative bacilli e. how often to meet to evaluate outcomes f. how to record the outbreak: infected and uninfected persons, place and time 3. Notify hospital and public health authorities if necessary 4. Institute necessary infection control interventions 5. Decide on frequency of regular meetings to assess the extent of outbreak 6. Meet regularly and assess progress 7. Decide when outbreak has ended |
Table 21.2 Usual modes of spread of outbreaks, including environmental sources.
| Organism | Usual mode of spread | Possible environmental sources |
| Acinetobacter | Hands of staff | Parenteral nutrition |
| Burkholderia | Hands of staff | Sinks, humidifiers, CVCs |
| Enterobacter cloacae | Hands of staff | Multiple dose medications |
| Enterobacter sakazakii | Powdered milk formula | |
| Klebsiella | Hands of staff | IV fluids, breast pump, disinfectant |
| MRSA | Hands of staff | Staff nasal carriage (‘cloud adults’) |
| Pseudomonas | Hands of staff | Sinks, taps/faucets, water bath, hand lotion |
| Salmonella | Hands of staff | Resuscitator, thermometer |
| Serratia | Hands of staff | Soap, shampoo, breast milk, breast pump, feeds, etc. (see Section 26.6.1) |
| Staphylococcus aureus | Hands of staff | Staff nasal carriage (‘cloud adults’) |
| Vancomycin-resistant enterococci | Hands of staff | |
| CVC, central venous catheter; IV, intravenous; MRSA, methicillin-resistant S. aureus. | ||
21.6 Outbreaks due to non-fermentative Gram-negative bacilli
21.6.1 Pseudomonas
Most outbreaks of P. aeruginosa infections occur due to transmission on the hands of staff,35 and unless there is a clear risk factor, intensive investigations for environmental sources are not recommended immediately. However, Pseudomonas are water-loving organisms and outbreaks of Pseudomonas outbreaks have been linked to water outlets including tap water which can be both the result and cause of hand colonization,35,36 faucets (taps)37,38 including electronic faucets,38 and sinks.39 Because organisms are transmitted from water outlets on the hands of staff, improved hand hygiene may suffice to terminate the outbreak. Other outbreaks of P. aeruginosa infection have been linked epidemiologically to a water bath used to thaw fresh frozen plasma,40 contaminated hand lotion41 and a contaminated blood gas analyser.42
Staff colonization may contribute to Pseudomonas outbreaks and should be considered. In an investigation of endemic P. aeruginosa on an NICU, staff with artificial fingernails and staff with nail wraps were more likely to have colonized hands and a health-care worker with onychomycosis carried the epidemic strain.43 Another outbreak was linked to a staff member with recurrent otitis externa with P. aeruginosa.44
Feeds may rarely cause Pseudomonas outbreaks. An outbreak of P. aeruginosa in a Spanish NICU was caused by contaminated feeding bottles prepared in the hospital feeding room.45 In France an outbreak of 31 cases of P. aeruginosa infection including four deaths was caused by a contaminated milk bank pasteurizer and bottle warmer.46
Neonatal P. aeruginosa bacteraemia has a >50% mortality (see Section 14.2.7). Although most infants colonized with P. aeruginosa do not develop sepsis, it is an unwelcome colonizing organism on neonatal units. Identifying one colonized infant should raise consideration of precautions (isolating the infant and screening other infants for carriage) to prevent a possible outbreak.
Environmental pseudomonads are often contaminants and can sometimes cause pseudo-outbreaks or outbreaks of pseudo-bacteraemia. In one such pseudo-outbreak, multi-resistant pseudomonads were cultured from several infants using in-house culture media but not from commercial media. The in-house media, stored under the sink, was thought to have been contaminated by water splashed from the sink.47 A pseudo-outbreak should be suspected when environmental pseudomonads are isolated in blood cultures from relatively well infants. However, environmental pseudomonads can cause central-line-associated infections occasionally. Reported outbreaks of infections with environmental pseudomonads may have been true or pseudo-outbreaks.48
21.6.2 Acinetobacter
Acinetobacter are non-motile, non-fermenting, aerobic Gram-negative coccobacilli found in soil and fresh water.49 Outbreaks of infection on neonatal units are generally thought to be transmitted on the hands of staff.50,51 A systematic review of invasive Acinetobacter infections described 18 outbreaks, 16 of them in neonatal units, mostly causing bacteraemia and sometimes meningitis.52 The outbreaks were caused by multiple different species, mainly A. baumannii (6 outbreaks) and A. calcoaceticus (5). Multi-drug resistant strains are increasingly reported.50,51 Many studies describe isolation of Acinetobacter from environmental sources including air conditioners, suction catheters and dressings and hands of health-care workers, but typing was not usually performed to confirm whether patient and environmental strains were identical.52 In the only study which clearly showed that an environmental source caused an outbreak, an Acinetobacter strain was isolated from total parenteral nutrition solution.53An outbreak with a highly resistant Acinetobacter strain should trigger review of the empiric antibiotic regimen, which may need changing until the outbreak resolves.51,54
21.6.3 Burkholderia cepacia
Neonatal outbreaks of B. cepacia have been linked with central venous catheters55 and B. cepacia has been grown in environmental samples from the water in a delivery room oxygen humidifier; from ventilator water traps and a humidifier water trap in the neonatal unit;54 and from sinks.56 It is an environmental organism which can also cause pseudo-outbreaks of bacteraemia (or outbreaks of pseudo-bacteraemia),57,58 including one associated with a contaminated blood gas analyser.57 Junior doctors collecting precious blood samples sometimes use the same syringe to inject blood into a blood gas analyser and then into blood culture bottles, causing pseudo-outbreaks.
21.7 Outbreaks due to Enterobacteriaceae
The Enterobacteriaceae are an important cause of nosocomial infections and of neonatal unit outbreaks. As their name suggests they are enteric organisms although can colonize the respiratory tract. They can all carry plasmid genes coding for extended spectrum β-lactamases (ESBLs).59
21.7.1 Serratia marcescens
S. marcescens is a hardy Gram-negative bacillus. The organism is usually transmitted via the hands of staff,60,61 but outbreaks have been linked with contaminated soap,62–64 hand-washing brushes,65 baby shampoo66 and multi-dose bottles of liquid theophylline.67 Outbreaks have been associated with feeds using contaminated milk bottles,67 enteral feed additives68 and breast pumps.69,70 Serratia produces a red pigment (which has been described to be a cause of the appearance of apparent blood-stained ‘stigmata’ on religious statues). S. marcescens may be grown from breast milk,71 including one mother who reported her breast pump tubing had turned bright pink.72 Contaminated delivery room tocogram transducers were the suspected source in one outbreak67 and laryngoscopes73 in another. Serratia was isolated from air conditioning ducts during one outbreak although the mode of spread was unclear.74
A systematic review of 34 outbreaks of S. marcescens in NICUs and PICUs, using genotyping to determine clonality, concluded that two or more temporally related cases of nosocomial infection should raise the suspicion of an outbreak.59 The authors recommended enhanced infection control measures, including nursery screening of infants (with regular stool samples) and cohorting of colonized infants, as the initial outbreak response.75 Environmental sampling from likely sources is only recommended if enhanced infection measures fail to contain the outbreak. An outbreak with a multi-resistant organism should trigger re-evaluation of empiric antibiotic treatment, in consultation with a paediatric infectious disease specialist or microbiologist.59
21.7.2 Enterobacter
E. cloacae is the major cause of reported Enterobacter outbreaks. A review of 26 outbreaks found that most were associated with over-crowding but found no common source, implying transmission on hands of staff. Two outbreaks were related to multiple-dose medications.76
E. sakazakii is an organism especially associated with contamination of powdered milk formula. It was recognized as a separate species in 1980 having previously been classified as a yellow-pigmented E. cloacae. It has recently been re-classified as Cronobacter sakazakii. It has been cultured from powdered milk formula in many different Western and developing countries.77 It can cause isolated cases of sepsis, outbreaks of bacteraemia and meningitis78,79 and outbreaks of necrotizing enterocolitis.80 A single case occurring in a bottle-fed infant should alert the clinician to the possibility of contaminated infant formula. Breastfeeding is protective and nosocomial Enterobacter infection is rarely described in exclusively breastfed infants.81
21.7.3 Klebsiella
Most outbreaks with Klebsiella species are linked to poor infection control and resolve with improved hand washing and other infection control measures,82 suggesting spread on the hands of staff is the most common mode of transmission.83
Outbreaks have been described in association with contaminated dextrose-containing intravenous fluids.84–86 One outbreak was associated with a contaminated breast milk pump87and one with contaminated disinfectant.88
21.7.4 Escherichia coli
E. coli can cause devastating outbreaks of neonatal diarrhoea in infant wards, although rarely described in neonatal units. E. coli are an important cause of early-onset and late-onset neonatal infections in developing countries89 but outbreaks with infant-to-infant spread have not been reported.
21.7.5 Salmonella
Most neonatal Salmonella infections are transmitted from the mother at the time of delivery but the organism can be spread readily to other infants, presumably on the hands of staff. In developing countries devastating outbreaks of invasive neonatal Salmonella infection have been repeatedly traced to contaminated suction machines in the delivery room90–92 or the neonatal unit.92 Salmonella outbreaks have also been described in Western countries. One outbreak was traced to an asymptomatic infected mother with spread via a resuscitator in the labour ward operating theatre.93 In another, Salmonella, spread from a mother and her child via inadequately disinfected thermometers in the labour suite and ward.94
21.8 Outbreaks due to Staphylococcus aureus
Infant-to-infant spread of S. aureus commonly occurs on the hands of staff.95 Early recognition of bacteraemia is associated with improved outcome.96
Improved hand hygiene, screening infants for carriage, and cohorting colonized infants are the main approaches to controlling outbreaks.95–103 Other more intensive infection control measures may need to be instituted.95–103 Topical mupirocin is often recommended to eradicate carriage in staff and infants, but has not been studied formally in outbreaks and is not always successful.100
Investigation of staff is controversial. Outbreaks have been associated with infected eczema of the hands or ears (otitis externa) of staff. During an outbreak, any infected skin lesions should be cultured and referral for formal dermatologic assessment considered.
Airborne transmission has had a possible role in some outbreaks. The first report of ‘cloud babies’ in 1960 described increased airborne dissemination of S. aureus in babies with respiratory tract co-infection with viruses (adenovirus or enterovirus).104 Some outbreaks of S. aureus were linked to staff with nasal carriage. The possibility of ‘cloud adults’ is supported by experiments in which nasal carriers of S. aureus given experimental rhinovirus infection developed increased staphylococcal shedding.105,106 However, the clinical significance and the need to screen staff for nasal carriage during S. aureus outbreaks remains uncertain. One recommendation is to assess and improve existing infection control measures first and only investigate staff nasal carriage if unsuccessful.
21.9 Outbreaks due to vancomycin-resistant enterococci
Neonatal outbreaks of vancomycin-resistant enterococci (VRE) infection are rare. Carriage is far more common than infection.107–113 VRE carriage has been associated with prolonged use of vancomycin and prolonged hospital stay.110 Published recommendations on prevention emphasize avoiding vancomycin use and good infection control practices, particularly screening for faecal carriage, cohorting colonized infants and rigorous infection control measures (Section 21.2.1).114,115
21.10 Bordetella pertussis
Although high levels of maternal antibodies protect against neonatal pertussis infection,116 most neonates are susceptible to infection with B. pertussis. The morbidity and mortality of pertussis is higher in neonates and infants <;3 months old than at any other age.117 The incubation period is 5–10 days. Infected neonates are usually afebrile and may present with apnoea without cough, although some develop increasingly severe paroxysmal cough. Whoop may or may not be present but colour change to red or purple during coughing paroxysms is classic and vomiting common.117
The source of infection is only identified in about half of all non-hospitalized infants with pertussis, usually a parent, more commonly the mother, or a sibling.118–123 In hospitalized infants, however, members of staff are an important and often unrecognized source of infection. Infected infants do not have a strong cough so, although infected infants should be placed in strict respiratory isolation to protect other infants, diagnosing infected family and staff members arguably provides better protection.124 Prevention is better than cure. Mathematical modelling suggests immunizing staff with a booster dose of pertussis vaccine would decrease the probability of secondary transmission from 49% without boosting to 2% if 95% of staff are boosted, and will decrease the final size of an outbreak.125 It has been estimated that a programme to give staff a booster dose of acellular pertussis vaccine would be cost-effective at anything over 25% uptake.126 Neonatal pertussis immunization is considered in Chapter 23.
A Cochrane systematic review of 13 trials of antibiotics in pertussis found short-term macrolide antibiotics (azithromycin for 3–5 days or clarithromycin or erythromycin for 7 days) were as effective as long-term (erythromycin for 10 days) at eradicating nasopharyngeal B. pertussis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


