Fig. 1
Steroid regimen as proposed by the Childhood Arthritis and Rheumatology Research Alliance for juvenile systemic lupus erythematosus
In maintenance therapies, steroids are usually continued at a low dose (2.5–5 mg/day). In the Hopkins Lupus Cohort, 57 % of patients with disease duration over 10 years have never discontinued steroids [3].
Other jSLE Lesions
Except for skin involvement, steroids remain the mainstay of treatment for other organ lesions in SLE. In the case of joint involvement, methotrexate can be proposed as a steroid-sparing agent. Azathioprine has been widely used to manage cytopenia, myositis, hepatitis, vasculitis, and nephritis [9]. MMF seems to be safe and has a similar efficacy to that in renal involvement [10]. Plasma exchange is of minor interest in SLE and may be occasionally useful in refractory antiphospholipid antibody syndrome.
Burden of Steroid Use in Pediatric-Onset SLE
General
Steroids constitute a significant source of morbidity in patients with lupus. Brunner et al. reported that children accumulated disease damage at almost twice the rate of adults and that long-term use of high-dose corticosteroids contributes to this disease damage [11].
In the Hopkins Lupus Cohort Study, corticosteroid-related damage was assessed in 539 patients including 18 with pediatric-onset SLE [3]. Osteoporotic fracture, coronary artery disease, cataracts, stroke, diabetes mellitus, and avascular necrosis were significantly associated with the cumulative corticosteroid dose. High-dose corticosteroid therapy contributes greatly to therapy-related damage in children.
Growth and Puberty
In a study by the Paediatric Rheumatology International Trials Organization (PRINTO), growth and puberty were evaluated in 1,015 children with SLE. Growth failure and delayed puberty were observed in 15.3 % and 11.3 %, respectively [12]. In another work, Rygg and colleagues highlighted that the negative effects of steroids on height and pubertal development were most pronounced in prepubertal and peripubertal children treated with over 400 mg/kg cumulative dose of GC [13].
Osteoporosis
Occurrence of osteopenia in patients with childhood-onset SLE has been well documented in studies assessing bone mineral density by dual-energy X-ray measurement [14–16]. Osteoporosis in jSLE is secondary to the cumulative effects of chronic inflammation, pubertal delay, renal failure, sustained steroid intake, and decreased sun exposure. The cumulative GC dosage is reported to be independently associated with decreased bone mass in patients with pSLE [16].
There are two leading mechanisms by which steroids induce osteopenia:
1.
Osteoclastogenesis induction with increased expression of RANK ligand and lower expression of its decoy receptor osteoprotegerin
2.
Inhibition of osteoblastogenesis by induction of osteoblast apoptosis and growth factor inhibition [17]
Keeping GC down to the lowest dose and using steroid-sparing agents are mandatory to avoid osteoporosis. In addition, vitamin D and calcium supplementation is required but guidelines are missing. Bisphosphonate use may be effective for restoring bone density, even in patients without fractures [18].
Dyslipidemia, Metabolic Syndrome, and Cardiovascular Complications
Steroids are considered a risk factor for atherosclerosis because they may induce hyperlipidemia, hyperglycemia, hypertension, and obesity in addition to being an independent risk factor for cardiovascular disease, which suggests that they may promote atherogenesis [19]. Given their lifelong exposure to atherogenic risk factors, children and adolescents with SLE are at a particularly high risk of developing premature atherosclerosis. The Atherosclerosis Prevention in Paediatric Lupus Erythematosus trial showed that atorvastatin may be effective in reducing carotid intima medial thickness progression in patients with pSLE in pubertal age with high C-reactive protein levels [20].
Infections
Mortality in the initial few years of disease is mainly associated with infections, resulting both from the use of immunosuppressants and from the SLE-related susceptibility to infections [21]. In the early phase of treatment or in the case of persisting lymphopenia, antibiotic prophylaxis with cotrimoxazole is advised.
Others
Hirsutism, moon facies, buffalo hump, acne, striae, and weight gain are additional side effects of steroids. These side effects have an impact on the self-esteem of adolescents and consequently on medication adherence.
New Drug Strategies in jSLE
In CTDs and especially in SLE, new drug regimens are now considered and aim at targeting more specifically the defective immune pathway and at sparing steroid use [22, 23].
Tacrolimus is a T cell-specific calcineurin inhibitor that prevents transcription of the early activation genes of interleukin-2 and suppresses T cell-induced activation of cytokines. Recent studies in adult-onset SLE have found tacrolimus to be effective and relatively safe [24–26]. Multitarget therapies including steroids, low-dose tacrolimus, and mizoribine – an inhibitor of purine nucleotide synthesis similar to MMF – have been tested in children with lupus nephritis (Class III, IV, and/or V) with good efficacy [27].
In renal transplantation, the use of induction regimens with depleting antibodies has allowed steroid dosing to be avoided or dramatically reduced. The LUNAR study, a randomized controlled trial, has compared high-dose steroids plus MMF with high-dose steroids plus MMF plus rituximab. This study did not reveal any benefit of rituximab as an add-on therapy [28]. Lighstone and colleagues in an observational single-center study proposed the rituxilup protocol, using rituximab and methylprednisolone on day 1 and day 15, associated with daily MMF treatment with a good response [29, 30]. This study and others suggest that rituximab may be used as a steroid-sparing strategy in lupus nephritis. Further controlled studies including pediatric patients are ongoing.
Belimumab is an antibody directed against B cell-activating factor. Although it has been approved by the Food and Drug Administration for treatment of active non-renal adult-onset SLE, data in children are lacking [31, 32]. It may represent an interesting add-on therapy in juvenile SLE. Current clinical trials assessing safety and pharmacokinetics are ongoing in children.
Steroids in Other CTDs
Juvenile Dermatomyositis
Treatments for juvenile dermatomyositis or other inflammatory myopathies have not been assessed in randomized controlled trials [33]. As in pSLE, treatment relies on steroids. In daily practice, steroids are often used for the first 2 years of treatment. Early initiation of high-dose steroids seems to be associated with a decreased incidence of calcinosis [34]. In addition, gut vasculitis may impact on oral steroid absorption, and some physicians treat patients with repeated pulses of high-dose IV methylprednisolone in addition to low-dose daily oral corticosteroid as this strategy can be cost-effective and may be associated with an earlier remission [35]. Delayed or inadequate corticosteroid treatment is one of the most important predictors of poor outcome with decreased bone density and chronic active skin lesions [15, 34, 36]. Initial treatment plans have also been proposed by the CARRA for juvenile dermatomyositis (Fig. 2).
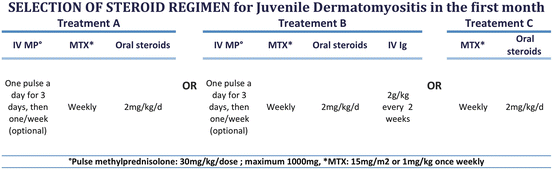

Fig. 2
First 4 weeks of treatment in juvenile dermatomyositis
Mixed Connective Tissue Disease
Mixed connective tissue disease is rare in children, accounting for less than 1 % of pediatric rheumatology patients in one series [37]. Steroids were used in 71 % of patients, depending on the affected organs [38]. Currently, there are no guidelines on the steroid regimen and most of the strategies are linked to the clinical spectrum/overlapping symptoms.
Juvenile Scleroderma
There are two main forms of the disease: juvenile localized scleroderma (JLS) and juvenile systemic sclerosis. The steroid administration mode highly depends on the specialty of the provider. In JLS, dermatologists prescribe topical steroids in 68 % and methotrexate in 4 % of patients, whereas rheumatologists conversely treat with local steroids in 4 % of cases and methotrexate in 38.8 % [39]. The CARRA group also proposed three distinct steroid regimens for JLS [40]: one regimen with methotrexate only, a second regimen with methotrexate + IV corticosteroids (either three consecutive daily doses/month for 3 months or one pulse/week × 12 weeks), and a third with methotrexate and oral prednisone (from 2 mg/kg/day initially to 12.5 % of initial dose at week 24).
Conclusions and Perspectives
Overall survival in CTDs has improved during the past few decades. Steroids are particularly effective in controlling inflammation during disease flares and for this reason they remain the cornerstone of treatment of CTDs. Alternative therapies, such as multitarget strategies or targeted therapies may help to decrease cumulative exposure to steroids, especially for remission induction. Treatments are mostly empirical and controlled trials in pediatric-onset CTDs are lacking. Further studies are highly warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


