Fig. 2.1
Schematic depiction of the possible role of interaction between several factors involved in the formation of the hypoxic placenta and its role in RPL. As shown, most complications are related to very early events in pregnancy. The abnormal genetic information influenced by environmental hazard and several immunological events lead to abnormal cross talking information during pre- and implantation period the Abnormal activation of placental biological molecules (growth factors/cytokines hormones and enzymes that lead to Abnormal activation of placental biological molecules growth factors cytokines, hormones, enzymes Elevatuon of d circulating biological molecules Clinical manifestations of: Abortion, PET, IUFD, IUGR and RPL
In this chapter only a few pitfalls related to RPL will be discussed. However, these events play a significant role in the development of normal pregnancy. Pre-implantation, vascularization, invasion, and oxidative stress are the main players in the regulation and function of these events and are the leading elements in good pregnancy outcomes. It seems that the mechanisms of RPL are dependent more on the maternal genetic predisposition (activation or silencing of several genes) than on the fetal chromosomal count [2].
Preimplantation
Human reproduction is characterized by a high incidence of peri-implantation loss [3]. The highly dynamic nature of the human endometrium is well documented. In response to the rise and fall in ovarian hormones, it proliferates, differentiates, sheds, and regenerates approximately 400 times during reproductive years. This process of continuous reshaping and regeneration of the endometrium depends on the presence of adult stem cells, migratory resident cells, coordinated influx of specialized immune cells, controlled inflammation, and angiogenesis [4]. Increasing evidence suggests that some women may experience RPL when a “super-receptive” endometrium allows embryos of low viability to implant, presenting as a clinical pregnancy before miscarrying [5–7].
The concept of super-receptivity is supported by the recent observation of a reduced interval between pregnancies in women with RPL compared to that reported by normally fertile women [8]. Further evidence comes from studies demonstrating lower levels of endometrial mucin-1, an anti-adhesion molecule that contributes to the barrier function of the epithelium in women with RPL [6]. Moreover, endometrial stromal cells (H-EnSCs) of women with RPL demonstrate abnormal decidualization in vitro [7]. This phenotype may result in the window of implantation being extended [7] (Fig. 2.2), while reducing the ability of the decidualized endometrium to be “selective” in response to embryo quality [9]. This concept is consistent with the previously reported association between implantation occurring later in the luteal phase and preclinical pregnancy loss [10].
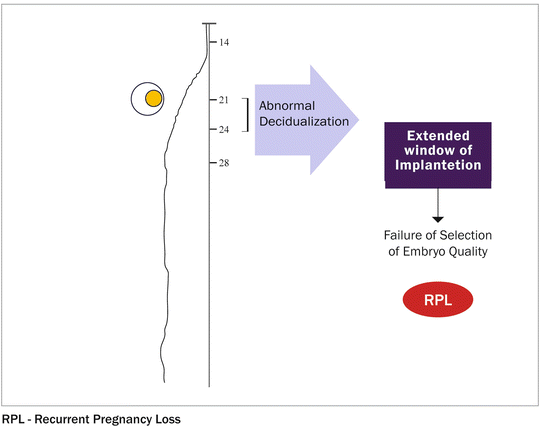

Fig. 2.2
Receptivity window—defined as the stage of endometrial maturation when the blastocyst can become implanted. Must be synchronized with embryo development and defined as the human window of receptivity—days 20–24 of a 28-day cycle. An intimate cross-talk between the embryo and the uterus is needed for blastocyst implantation. This process, which consists of an interaction between trophoblast cells and endometrium, is initially dependent on the presence of estrogen and progesterone, although further morphological and biochemical changes are evoked within the uterine wall by signals from the embryo and invading trophoblast. MLCP, resulting in actomyosin contraction. Detachment of the rear end: focal contacts disassemble and integrins detach from the substrate. Invasion denotes cellular movement within tissues and requires degradation of
The preparatory process for pregnancy starts with the postovulatory surge in circulating progesterone levels. This process inhibits estrogen-dependent proliferation of the uterine epithelium and induces secretory transformation of the uterine glands. Subsequently, the luminal epithelium expresses an evolutionarily conserved repertoire of molecules essential for stable interaction and adherence of a blastocyst, thus enabling implantation. The receptivity is a transient endometrial state, confined to only a few days in the mid-secretory phase of the cycle, and depends on paracrine signals from decidualizing endometrial stromal cells (ESCs) underlying the luminal epithelium [11]. This process is defined by mesenchymal-to-epithelial transformation of endometrial fibroblasts into secretory decidual cells [12]. Decidualization is indispensable for pregnancy as it confers immunotolerance to the fetal semi-allograft, controls trophoblast invasion, and both nourishes and protects the peri-implantation conceptus against a variety of physiological stressors associated with pregnancy [13]. The decidual ESCs operate as gatekeepers of different immune cells at the implantation site. The differentiating ESCs secrete interleukin-11 (IL-11) and IL-15, implicated in recruitment and differentiation of uterine natural killer (NK) cells, which in turn are a rich source of angiogenic factors [14–16]. In most species, the implanting embryo triggers the decidual process. In humans, however, decidualization is under maternal control and is initiated during the mid-secretory phase of each menstrual cycle in response to elevated progesterone and rising cellular cAMP levels [17]. ESCs first mount an acute auto-inflammatory response upon decidualization, which in turn triggers the expression of key receptivity genes in the overlying endometrial surface epithelium [18]. This pro-inflammatory phenotype is transient, determines the duration of the window of implantation, and is followed by an anti-inflammatory response essential for post-implantation embryo support and coordinated trophoblast invasion [18] (Fig. 2.2). In the absence of pregnancy, falling progesterone levels reactivate the expression of inflammatory mediators in decidualizing ESCs, triggering apoptosis, influx of immune cells, extracellular matrix (ECM) breakdown, and menstrual shedding [19, 20]. An inevitable consequence of menstruation is the need for cyclic regeneration and renewal of the endometrium. The regenerative capacity of the human endometrium is indeed remarkable. It is rich in mesenchymal stem-like cells (MSCs) residing predominantly around the vessels. They are recruited to the endometrium in response to hypoxic, proteolytic, and inflammatory stimuli associated with cyclic menstruation, pregnancy, and parturition. In fact, purified ESCs also express elevated levels of pluripotency factors, and are more agreeable to induced pluripotent stem cell reprogramming compared with conventional somatic cells. Molecular phenotyping indicates that ESCs are closely related to follicular dendritic cells (FDCs). The ESCs and FDCs both originate from perivascular platelet-derived growth factor receptor β-positive (PDGFRβ+) adult stem/precursor cells, differentiate in response to inflammatory signals, and are key to local and systemic maternal immunotolerance in pregnancy, respectively [21]. New data demonstrate that coordinated migration and invasiveness of decidualizing ESCs in response to embryonic and trophoblast signals are key to successful implantation. In addition, endometrial cells are capable of invading distant sites, leading to pelvic endometriosis or uterine adenomyosis [22, 23].
Migration and Invasion of Trophoblast
The migratory and invasive capacity of mature ESCs and progenitor cells is increasingly recognized to support the intense tissue remodeling associated with endometrial regeneration, decidualization, embryo implantation, and trophoblast invasion. The lack of fine-tuning of ESC migration and invasion and deregulation of these cell functions contributes to common reproductive disorders, such as implantation failure and recurrent pregnancy loss (RPL).
Cellular Movement
Cellular movement in response to a signal can be classified into two major types: chemokinesis and chemotaxis. Chemokinesis occurs when a factor stimulates cell motility without determining the direction of migration; chemotaxis takes place when cells migrate toward a chemoattractant in a concentration gradient [24]. Chemokinesis is a random and nondirected type of migration, whereas chemotaxis is directed locomotion in response to an external cue.
Implantation
Implantation , a critical step for the establishment of pregnancy, requires complex molecular and cellular events resulting in uterine growth and differentiation, blastocyst adhesion and invasion, and placental formation. Successful implantation necessitates a receptive endometrium, a normal and functional embryo at the blastocyst stage, and a synchronized dialogue between the mother and the developing embryo [25]. In addition to the well-characterized role of sex steroids, the complexity of blastocyst implantation and placentation is exemplified by the role played by a number of cytokines and growth factors in these processes. Indeed, the process of implantation is orchestrated by hormones such as sex steroids and hCG; growth factors such as TGF-B, HB-EGF, and IGF-1; cytokines such as Leukemia Inhibitory Factor, Interleukin-6, and Interleukin-11; adhesion molecules including L-selectin and E-cadherin; the extracellular matrix (ECM) proteins; and prostaglandins [25]. Embryonic implantation is initiated by the recognition and adhesion between the blastocyst surface and the uterine endometrial epithelium. Adhesion occurs when a free-floating blastocyst comes into contact with the endometrium during the “receptive window” during which it is able to respond to the signals from the blastocyst. This contact is then stabilized in a process known as adhesion, in which the trophoblast cells establish contact with the micro-protrusions present on the surface of the endometrium known as pinopodes [26]. The last step of implantation is the invasion process, which involves penetration of the embryo through the luminal epithelium into the endometrial stroma; this activity is mainly controlled by the trophoblast [27]. The trophoblast lineage is the first to differentiate during human development, at the transition between morula and blastocyst. Initially, at day 6–7 post-conception, a single layer of mononucleated trophoblast cells surrounds the blastocoel and the inner cell mass. At the site of attachment and direct contact to maternal tissues, trophoblast cells fuse to form a second layer of post-mitotic multinucleated syncytiotrophoblast [28]. Once formed, the syncytiotrophoblast grows by means of steady incorporation of new mononucleated trophoblast cells from a proximal subset of stem cells located at the cytotrophoblast layer [29]. Tongues of syncytiotrophoblast cells begin to penetrate the endometrial cells, and gradually the embryo is embedded into the stratum compactum of the endometrium. A plug of fibrin initially seals the defect in the uterine surface, but by days 10–12 the epithelium is restored [30]. Only at around the 14th day do mononucleated cytotrophoblasts break through the syncytiotrophoblast layer and begin to invade the uterine stroma at sites called trophoblastic cell columns. Such cells constitute the extravillous trophoblast and have at least two main subpopulations: interstitial trophoblast, comprising all those extravillous trophoblast cells that invade uterine tissues and that are not located inside vessel walls and lumina; and endovascular trophoblast, located inside the media or lining the spiral artery lumina and partly occluding them (sometimes this subtype is further subdivided into intramural and endovascular trophoblasts) [30]. At a molecular level, trophoblast adhesion from the stage of implantation onwards is an integrin-dependent process [31] that takes place in a chemokine- and cytokine-rich microenvironment analogous to the blood-vascular interface. Of note, uterine expression of chemokines in humans is hormonally regulated and the blastocyst expresses chemokine receptors. In addition, oxygen tension plays an important role in guiding the differentiation process that leads to cytotrophoblast invasion of the uterus [32].
The Selectins Adhesion System
The selectins adhesion system and cadherin families are the main adhesion molecules investigated with regard to the implantation process. Selectins are a group of three carbohydrate-binding proteins that are named following the cell type expressing them (E—endothelium, P—platelets, and L—leucocytes): E-selectin is expressed on the endothelial surface; P-selectin on the surface of activated platelets; and L-selectin on lymphocytes, where it plays an essential role in the homing mechanism of these cells [27, 33, 34]. Transmigration may constitute an initial step in the implantation process. Indeed, L-selectin is strongly expressed on the blastocyst surface while, during the window of implantation, there is an upregulation in the decidual expression of the selectin oligosaccharide-based ligands, predominantly on endometrial luminal epithelium [35]. This may assist in the blastocyst decidual apposition during the implantation process. The effect of heparin on selectins during implantation is unclear. Due to its high density in negatively charged sulfates and carboxylates, heparin is able to bind the two binding sites of the natural ligand of selectin molecules (P- and L-selectins: one for the sialyl Lewis X moiety and another for the tyrosine sulfate-rich region of its native ligand P-selectin glycoprotein ligand-1 [PSGL-1]); the number of sites bonded is dependent on the length of the heparin chain. Evidence in support is presented by the study of Stevenson, Choi, and Varki [36], who investigated the effect of different unfractionated heparin and LMWH on selectin molecules in cancer cell lines [27]. Tinzaparin, with 22–36 % of its fragments greater than 8 kDa, significantly impairs L-selectin binding to its ligand; whereas enoxaparin, with 0–18 % fragments greater than 8 kDa, did not affect L-selectin expression [36]. Thus, heparins with a high proportion of fragments longer than 8 kDa may reduce inflammatory cell adhesion and homing; on the other hand, they may affect blastocyst adhesion by blocking selectin ligand binding sites. Cadherins are a group of cell adhesion proteins that mediate Ca2+-dependent cell–cell adhesion, a fundamental process required for blastocyst implantation and embryonic development [37]. E-cadherin plays an important role in maintaining cell adhesion. In cancer cells, the reduction of E-cadherin expression promotes acquisition of an invasive phenotype. Remarkably, gestational trophoblastic diseases (choriocarcinoma and complete hydatidiform mole) that are characterized by invasive trophoblast behavior have a lower E-cadherin trophoblastic expression than that of first-trimester placenta. In contrast, the trophoblast expression of E-cadherin is higher in placentas of patients with preeclampsia than in those of normal pregnant women [38]. Evidence to support the effect of heparins on trophoblast invasiveness through E-cadherin expression provides a possible mechanism by which heparin could promote trophoblast cell differentiation and motility.
Heparin-Binding EGF-Like Growth Factor
Heparin-binding epidermal growth factor (EGF) -like growth factor (HB-EGF) is a 76–86 amino acid glycosylated protein that was originally cloned from macrophage-like U937 cells. It is a member of the EGF family that stimulates growth and differentiation. HB-EGF utilizes various molecules as its “receptors.” The primary receptors are in the ErbB (also named HER) system, especially ErbB1 and ErbB4 human tyrosine kinase receptors. HB-EGF is initially synthesized as a transmembrane precursor protein, similar to other members of the EGF family of growth factors. The membrane-anchored form of HB-EGF (proHB-EGF) is composed of a pro-domain followed by heparin-binding, EGF-like, juxtamembrane, transmembrane, and cytoplasmic domains. Subsequently, proHB-EGF is cleaved at the cell surface by a protease to yield the soluble form of HB-EGF (sHB-EGF) using a mechanism known as ectodomain shedding. sHB-EGF is a potent mitogen and chemoattractant for a number of different cell types. Studies of mice expressing noncleavable HB-EGF have indicated that the major functions of HB-EGF are mediated by the soluble form HB-EGF accumulates in the trophoblast [39] throughout the placenta. HB-EGF has a multiple role due to its cell-specific expression during the human endometrial cycle and early placentation, and high level expression in the first trimester [27]. The membrane active precursor functions as a juxtacrine growth factor and cell surface receptor. It has been demonstrated that HB-EGF promotes adhesion of the blastocyst to the uterine wall in a mouse in vitro system [40], suggesting a role for HB-EGF in embryo attachment to the uterine luminal epithelium. The majority of its biological functions are mediated by its mature soluble form. A major role in early stages of placentation is represented by cellular differentiation and consequent invasion of the uterine wall and vascular network. Several changes occur in the expression of adhesion molecules as cytotrophoblast differentiation proceeds, which results in pseudovasculogenesis or the adaptation by cytotrophoblasts to a molecular phenotype that mimics endothelium [41]. For example, during extravillous differentiation in vivo, integrin expression is altered from predominantly α6β4 in the villous trophoblast to α1β1 in cytotrophoblasts migrating throughout the decidual stroma [31] or engaging in endovascular invasion. Leach et al. [42] demonstrated the role of HB-EGF in regulating the conversion of human cytotrophoblasts into an invasive phenotype and the motility of these cells. This study demonstrated the ability of HB-EGF to induce “integrin switching” through intracellular signaling following ligation of HER tyrosine kinases, altering integrin gene expression to stimulate cytotrophoblast invasion at a molecular level. In addition to its effect on the invasive trophoblast phenotype, HB-EGF can affect cell motility. Indeed, cytotrophoblast motility was specifically increased by each of the EGF family members examined. The expression by cytotrophoblasts of each growth factor, as well as their receptors, suggests the possibility of an autocrine loop that advances cytotrophoblast differentiation to the extravillous phenotype. The ability of the HB-EGF molecule to prevent hypoxic-induced apoptosis plays a fundamental role in early stages of placentation.
Oxidative Stress in Early Pregnancy
During gestation, an adequate and efficient supply of nutrients and oxygen is vital for proper development of the fetus. These fetomaternal exchanges rely on adequate vascularization of both the maternal decidua and the fetus-derived placental villi [43]. In the human maternal decidua, vascular remodeling of the intramyometrial portion of the spiral arterioles occurs between the 10th and 12th week of gestation. This transformation is achieved by specialized placental cells, the cytotrophoblasts. During placentation, cytotrophoblasts that are present in anchoring villi generate multilayered columns of highly invasive extravillous trophoblasts that colonize the interstitium of the maternal decidua, the inner third of the myometrium, and the uterine blood vessels. This invasion results in the formation of the low-resistance vascular system that is essential for fetal growth [43]. This developmental period (10–12 weeks of gestation) is characterized by an important physiological switch in oxygen tension during the opening of the intervillous space. Before the ninth week of gestation, placental oxygen tension is low (20 mmHg), and after 10–12 weeks of gestation, it increases to approximately 55 mmHg [43]. At this time, the cytotrophoblast turns from a proliferative to an invasive phenotype [44]. Failure of this transition is associated with clinical complications of pregnancy, including preeclampsia, the most common cause of retarded fetal development [45]. (Fig. 2.3)
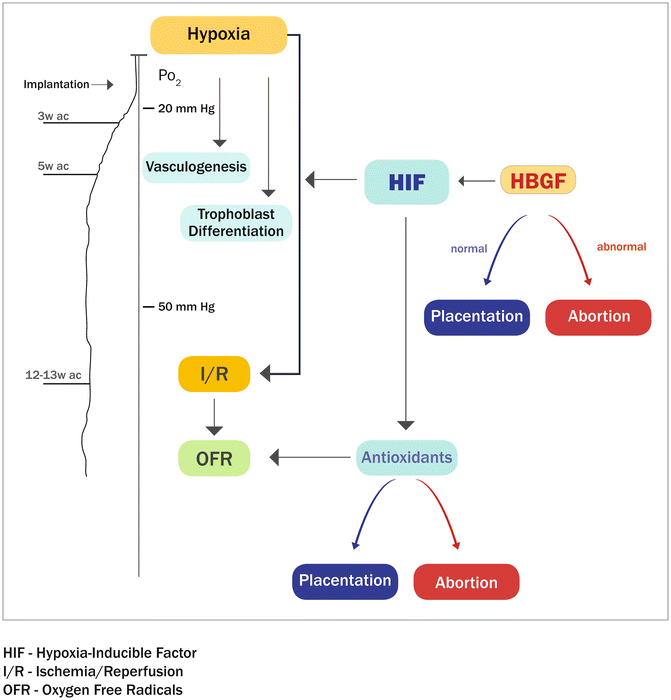

Fig. 2.3
Placental development is profoundly influenced by oxygen (O2) tension. Human cytotrophoblasts proliferate under low O2 conditions but differentiate at higher O2 levels. Trophoblasts must be able to accurately sense oxygen tension hypoxia-inducible factor-1 (HIF-1). HIF-1 activates many genes involved in the cellular response to O2 (also known as aryl hydrocarbon receptor nuclear translocator (ARNT)). HIF-1 is able to be stabilized under normoxic conditions by a variety of growth factors and cytokines including epidermal growth factor (EGF)
Hemochorial placentation is also dependent on the establishment and maintenance of a competent fetal placenta—a vascular network formed by branching (first and second trimesters) and nonbranching (third trimester) angiogenesis. In human placenta, branching angiogenesis is important for both the development of the villous vasculature and the formation of terminal villi. Consequently, both trophoblasts and endothelial cells are required during the early stages of placental development. Angiogenic growth factors are considered to be the main mediators of these processes. Mouse models have demonstrated the importance of two families of ligands, namely vascular endothelial growth factors (VEGFs) and angiopoietins and their tyrosine kinase receptors in fetal and placental angiogenesis [45]. Vasculogenesis starts during the third week after conception. This process is characterized by the formation of the first blood vessels from differentiation of pluripotent mesenchymal cells into hemangiogenic stem cells [45].
The subsequent step, angiogenesis, starts during the fifth week after conception and refers to the development of new vessels from preexisting vessels [45]. From day 32 to week 25 after conception, hemangioblastic cords formed by vasculogenesis develop into a richly branched villous capillary bed by two mechanisms: elongation of preexisting tubes and lateral ramification of these tubes (sprouting angiogenesis). Around week 25, this process switches from branching to nonbranching angiogenesis [45]. Nonbranching angiogenesis transpires in mid and late gestation and it is mainly characterized by endothelial cell proliferation leading to an increase in the surface of the endothelial tissue. These processes ensure the increasing supply of gas and nutrients for the growing fetus [45]. The existence of tissue-specific angiogenic factors has been postulated for many years [46], but only in the last decade has a factor, named endocrine gland-derived vascular endothelial growth factor/prokineticin 1 (EG-VEGF/PROK1), been characterized [46] as a trophoblast product.
The early placenta is poorly protected against oxidative damage. The antioxidant enzymes such as copper/zinc superoxide dismutase and mitochondrial superoxide dismutase are not expressed by the syncytiotrophoblast until approximately 8–9 weeks of gestation. The expression of the protective enzymes increases significantly after the trophoblast plugs are loosened and the placenta becomes exposed to gradually increasing levels of oxygen and consequently experiences oxidative stress [47]. At the end of the first trimester, a burst of oxidative stress is evidenced in the periphery of the early placenta. The underlying utero–placental circulation in this area is never plugged by the trophoblastic shell, allowing limited maternal blood flow to enter the placenta from 8 to 9 weeks of gestation. Focal trophoblastic oxidative damage and progressive villous degeneration trigger the formation of the fetal membranes, which is an essential developmental step enabling vaginal delivery. The oxidative stress and rise in oxygenation may also stimulate the synthesis of various trophoblastic hormones, such as hCG and estrogens. The oxidizing conditions promote assembly of the hCG subunits. Angiographic studies of the uterine vasculature have demonstrated that during normal pregnancy, flow from spiral arteries into the intervillous space is often intermittent, arising from spontaneous vasoconstriction. Placental inflow may also be compromised by external compression of the arteries during uterine contractions. Some degree of I/R (ischemia/reperfusion) stimulus may therefore be a feature of normal human pregnancy, especially toward term when the fetus and placenta are extracting large quantities of oxygen (O2) from the intervillous space. Chronic stimulus could lead to upregulation of the anti-OFR (oxygen free radicals ) defense in the placenta, reducing oxidant stress. In early pregnancy this well-controlled oxidative stress is critical in continuous placental remodeling and essential placental functions such as transport and hormonal synthesis. Miscarriages and preeclampsia could be a temporary maladaptation to a changing oxygen environment.
Independent of the cause of the miscarriage, the excessive entry of maternal blood into the intervillous space has two effects: a direct mechanical effect on the villous tissue, which becomes progressively enmeshed inside large intervillous blood thrombi, a widespread and indirect O2-mediated trophoblastic damage; and increased apoptosis. In preeclampsia the trophoblastic invasion is sufficient to allow early pregnancy phases of placentation too shallow for complete transformation of the arterial utero–placental circulation, predisposing to a repetitive ischemia–reperfusion (I/R) phenomenon. This would impair the placentation process, leading to chronic oxidative stress in the placenta and finally to diffuse maternal endothelial cell dysfunction.
Placental development is profoundly influenced by (O2) tension. Human cytotrophoblasts proliferate under low O2 conditions but differentiate at higher O2 levels. Trophoblasts must be able to accurately sense oxygen tension hypoxia-inducible factor-1. Hypoxia-inducible factor-1 (HIF-1), consisting of HIF-1 a-subunit and ARNT b-subunit (aryl hydrocarbon receptor nuclear translocator), activates many genes involved in the cellular response to O2 deprivation. To date, three members of the HIF family of transcription factors have been identified. All the members of the HIF family consist of an inducible alpha subunit (HIF-α) and a constitutively expressed beta subunit (HIF-β, also known as aryl hydrocarbon receptor nuclear translocator (ARNT)). HIF-1 is able to be stabilized under normoxic conditions by a variety of growth factors and cytokines including epidermal growth factor (EGF), insulin, heregulin, insulin-like growth factors 1 and 2, transforming growth factor ß1, and interleukin-1ß. There is evidence that antiphospholipid syndrome associated with direct inhibition of trophoblast invasion, rather than placental thrombosis, may be the reason for pregnancy loss. Defective decidual endovascular trophoblast invasion, rather than excessive intervillous thrombosis, was the most frequent histological abnormality in antiphospholipid syndrome-associated early pregnancy loss.
Mainly performed and published in vitro studies, related to placentation and implantation, are based on a concept that these processes are similar to malignant cell invasion and expulsion into surrounding tissues and histologic structures. Migratory and invasive capacity of human endometrial stromal cells (ESCs) is now recognized as a main process in early stages of human reproduction and contributes to the intense tissue remodeling associated with embryo implantation, trophoblast invasion, and endometrial regeneration. In this part of the chapter, we concentrate on the most interesting studies and ongoing experiments that have been published in scientific journals.
A prototypical stimulus for chemokinesis is the angiogenic platelet-derived growth factor (PDGF)-BB. When applied to cells in a homogeneous solution, PDGF-BB can also act asymmetrically as a chemoattractant. The main factors in the regulation of migration are Rho family small guanosine triphosphate (GTP)-binding proteins (GTPases). They control organization of the actin cytoskeleton and the formation of cellular protrusions. More than 20 members of the Rho GTPase family have been identified, including RhoA, Rac1, and CDC42. In their active GTP-bound state, Rho GTPases perform their function through effector proteins, e.g., Rho-associated serine/threonine kinase (ROCK). Rac1 and CDC42 initiate the formation of a branched actin filament network, whereas RhoA promotes linear elongation of actin filaments [47] (Fig. 2.4). A four-step model describes two-dimensional cell migration on biological surfaces [48, 49]: (a) Protrusion of the leading edge: growing actin filaments, controlled by Rac and CDC42 activity, push the cell membrane outward. (b) Cell matrix interactions and formation of focal contacts: integrins serve as receptors for ECM proteins, such as collagen, laminin, and fibronectin. Focal contacts are dynamic complexes linking ECM, integrins, and the cytoskeleton. Focal adhesion kinase (FAK) is recruited to these sites and regulates both their assembly and turnover. (c) Cell contraction by actomyosin: myosin light chain (MLC) is phosphorylated by MLC kinase and de-phosphorylated by MLC phosphatase (MLCP). The extent of MLC phosphorylation is regulated by RhoA through its effector ROCK, which phosphorylates and thus inhibits MLCP, resulting in actomyosin contraction. (d) Detachment of the rear end: focal contacts disassemble and integrins detach from the substrate. Invasion denotes cellular movement within tissues and requires degradation of the ECM. Focalized proteolysis by surface proteases generates active soluble matrix metalloproteinases (MMPs) with selected specificities for ECM components. Invasion further involves the action of cysteine- and serine-proteases, such as kallikreins and plasminogen activators (PA), and is counteracted by tissue inhibitors of MMPs (TIMPs) and PA inhibitor secreted by cells of the invaded tissue [50].
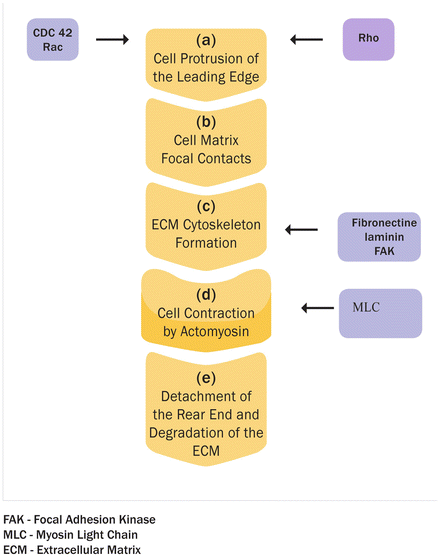

Fig. 2.4




Protrusion of the leading edge: Growing actin filaments, controlled by Rac and CDC42 activity, push the cell membrane outward. (b) Cell matrix interactions and formation of focal contacts: integrins serve as receptors for ECM proteins, such as collagen, laminin, and fibronectin. Focal contacts are dynamic complexes linking ECM, integrins, and the cytoskeleton. Focal adhesion kinase (FAK) is recruited to these sites and regulates both their assembly and turnover. (c) Cell contraction by actomyosin: myosin light chain (MLC) is phosphorylated by MLC kinase and de-phosphorylated by MLC phosphatase. (d) The extent of MLC phosphorylation is regulated by RhoA through its effector ROCK, which phosphorylates and thus inhibits MLCP, resulting in actomyosin contraction. (e) Detachment of the rear end: focal contacts disassemble and integrins detach from the substrate. Invasion denotes cellular movement within tissues and requires degradation of (a)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


