TVS
Terms and measurements
In the sagittal the endometrial thickness is measured
“Double endometrial thickness”
Intracavitary fluid: the thickness of both single layers are measured and the sum is recorded as the maximum measurement in the sagittal plane
Endometrial thickness
Amount of intracavitary fluid: largest measurement in the sagittal plane
Largest measurement of fluid
Echogenicity of fluid
Anechogenic, low-level echogenicity ground glass
Mixed echogenicity
Endometrial morphology
Endometrial echogenicity compared to the myometrium
Hyper-, iso- or hypoechogenic
Homogeneous with symmetrical anterior and posterior sides (includes the three-layer pattern, and the homogeneous hyper-, hypo- and isoechogenic endometrium)
Uniform
Heterogeneous, asymmetrical or cystic endometrium
Not uniform
The endometrial midline is described
Straight hyperechogenic interface
Linear
A waved hyperechogenic interface
Non-linear
Irregular interface
Irregular
Absence of a visible interface.
Not defined
The endometrial–myometrial junction
Regular, irregular, interrupted or not defined
An echo formed by the interface between an intracavitary lesion and the endometrium
Bright edge
Intracavitary pathology
Endometrial thickness including the lesion is measured (not including intracavitary myoma)
Intracavitary lesions should be measured in three perpendicular diameters (d1, d2, d3)
d1, d2, d3
The volume (V) of the lesion may be calculated from the three orthogonal diameters (d1 × d2 × d3 × 0.523)
V
Myoma measurement (Table 1.2)
Synechiae are defined as strands of tissue crossing the endometrium
Synechia
Color Doppler assessment of the endometrium
The color content of endometrium can be scored: (1) no color flow; (2) with minimal color; (3) moderate color; (4) abundant color is presented
Color score 1 to 4
Vascular pattern:
Scattered (dispersed color signals within the endometrium but without visible origin at the myometrial–endometrial junction) or not scattered vessels
Scattered, not scattered
Dominant vessel: one vessel passing the endomyometrial junction
Dominant, not dominant
Caliber of vessels
Large or small
Vessels may be single (double) or multiple, with focal or multifocal origin or there might be circular flow
Single (double), multiple focal, multifocal, circular flow
Branching of vessels
Orderly or disorderly/chaotic
GIS or SIS
The endometrial outline
Appears regular
Smooth
Multiple thickened “undulating” areas, “moguls” with a regular profile
Endometrial folds
Deep indentations or
Polypoid
Surface is cauliflower like or sharply toothed (“spiky”)
Irregular
Intracavitary lesions:
Extended: Lesion involves ≥ 25 % of the endometrial surface
Extended
Localized: Lesion involves < 25 % of the endometrial surface
Localized
Pedunculated: a/b ratio is <1; Sessile: : a/b ratio is ≥ 1
Pedunculated, sessile
a/b ratio between the diameter of the base level of the endometrium (a)
Maximal transverse diameter of the lesion (b)
The echogenicity of a lesion is defined as “uniform” (homogenic) or
Uniform
“Nonuniform” (heterogenic), which includes cystic lesions
Not uniform
The outline of the lesion is defined as “regular”
Regular
or “irregular” (e.g. spiky or cauliflower like)
Irregular
Table 1.2
Qualitative assessment of myometrium and myometrial lesions (myomas and adenomyosis)
Definition | Term, measurement | ||||||
|---|---|---|---|---|---|---|---|
Uterine contour | Normal: pear shape; globular: globally enlarged; bernoccolute: uterus with irregular external profile | Normal, globular, bernocculuto | |||||
Uterine volume | Length (d1), anterior posterior diameter (d2) and transverse diameter (d3) | (d1 × d2 × d3 × 0.523) | |||||
Uterine perimetrial outline | Regular: smooth with a regular shape; irregular: not smooth contour | Regular, irregular, not defined | |||||
Myometrial wall | Measurement of anterior and posterior wall thickness in sagittal plane | Symmetrical, asymmetrical maximal thickness of wall | |||||
Myometrial echogenicity | Homogenous | Uniform | |||||
Heterogenous, or cystic | Non-uniform | ||||||
Regular cystic or not regular cystic | Cystic | ||||||
Junctional zone | Hypoechogenic inner subendometrial halo | Regular, irregular, interrupted, not defined | |||||
Myometrial lesions | Presence, number, shape | ||||||
Contour | Clear hypo or hyperechoic external contour (rim) | Rim defined, not defined | |||||
Margins | Defined margins | Regular, irregular | |||||
Echogenicity | Hypo, iso, hyperechogenic (+/− shadows) | Uniform | |||||
Heterogenic mixed echo, cystic areas, lacunae, stripes, shadows | Not uniform | ||||||
Site | Anterior, posterior wall | ||||||
Location | Middle site or lateral (right, left, corneal or intra ligamentar) | ||||||
Fundus, corpus, isthmus, cervix | |||||||
The minimal distance between the perimetrium and the outer portion of myoma (dm). The minimal distance between the endometrium and the inner portion of the myometrium (de) | dm de | ||||||
Submucous myomas extension of the base: proportion of wall covered | |||||||
≤1/3; 1/3–2/3;>2/3 | |||||||
Type | Submucous (sm) | Type: 0,1,2 | |||||
(Type 0), myoma completely within the cavity; (Type 1) with ≥50 % of the endocavitary portion protruding into the cavity; and (Type 2), with the endocavitary part of myoma <50 % | |||||||
Other (O) | Type: 3,4,5,6,7,8 | ||||||
Contacts endometrium; 100 % intramural | Type 3 | ||||||
Intramural | Type 4 | ||||||
Subserosal ≥50 % intramural | Type 5 | ||||||
Subserosal <50 % intramural | Type 6 | ||||||
Subserosal pedunculated | Type 7 | ||||||
Other (Specify, e.g. cervical, parasitic) | Type 8 | ||||||
Hybrid impact on both endometrium and serosa | Type: 0–5 | ||||||
Size | The three largest perpendicular diameters (a1, a2, a3) mm | a1, a2, a3 | |||||
1/6 × π × a1 × a2 × a3 | Volume | ||||||
P = 6 A2 (B/2 –A/3)/B3 B largest diameter perpendicular to the uterine cavity and A part of this diameter in the uterine cavity | P = Proportion of myoma volume in uterine cavity | ||||||
Doppler morphology | Regular vessels or irregular vessels | Regular or irregular | |||||
Branching: regular, irregular, no branching | Regular branching | ||||||
Scattered dispersed color signal | Scattered | ||||||
Many vessels | Multiple pattern | ||||||
Caliper, number | Large, small, few, many | ||||||
Homogenic vessels, size | Homogenic | ||||||
Peripheral | Circular or not circular flow | ||||||
Color score: (1) no color flow; (2) with minimal color; (3) moderate color; (4) abundant color is presented | Color score 1–4 | ||||||
STEPW | Submucous classification for complexity of hysteroscopic surgery | ||||||
Score | Size | Topography | Extension of base | Myoma type | Lateral wall | ||
0 | <2 | Low | <1/3 | 0 (100 %) | +1 | ||
1 | 2–5 | Middle | 1/3–2/3 | 1 (>50 %) | |||
2 | >5 | Upper | >1/3 | 2 (<50 %) | |||
∑ | + | + | + | + | + | ∑ Total score (SUM) | |
SUM | 0–4 | Low complexity hysteroscopy | |||||
5–6 | High complexity hysteroscopy, two steps, medical preoperative treatment | ||||||
7–9 | Consider alternatives | ||||||
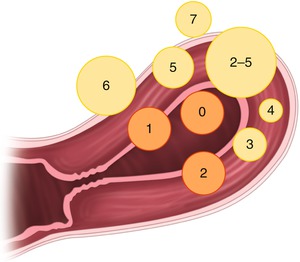
Fig. 1.1
In the FIGO system intracavitary myomas are classified by the traditional European Society for Gynaecological Endoscopy (ESGE) (type 0–2), and in addition intramural myomas are classified (type 3 to 7). Myomas with impact on both the endometrium and serosa are classified as type 2–5
After evaluation of the uterus, the remaining pelvic area should be evaluated. The ovaries and the uterine horns should be located in the transverse plan. The tubes can be followed from the uterine horns to the iliac vessels and these procedures will normally reveille the ovaries. Each ovary should be scanned in the transverse and sagittal plane from the bottom to top and scanning should be performed in two perpendicular planes. Areas above, beneath and besides the ovaries should be scanned at each side.
The ovaries should be described according to standard terms from the International Ovarian Tumor Analysis (IOTA) group (Timmerman et al. 2000, 2010) (Table 1.3). Again power Doppler may be added in the evaluation of abnormal findings. The urinary bladder and the pouch of Douglas should be evaluated. Sliding of different organs (gentle movement with the probe) should be noted. The urinary bladder, the rectum, and sacrouterine ligaments are also scanned and recorded.
Table 1.3
Terms in definition of ovaries and ovarian mass
Definitions | Measurements and terms | |
|---|---|---|
Size, site | Ovarian measurement: three perpendicular planes largest 3 diameters (mm) (a1, a2, a3) | a1,a2,a3 |
Ovarian volume | (a1 × a2 × a3 × 0.523). | |
Outline of ovary | Regular, irregular | |
Morphology of ovarian tissue | ||
Follikels (numbers (n) and size in largest perpendicular diameters (A1, A2, A3) (mm) | N, A1, A2, A3 | |
Antral follicle count | Count follicles between cycle days 2 and 4 | Antral follicle count |
Include all antral follicles of 2–10 mm in diameter (most reliable in 3D volume) in both ovaries | ||
Ovarian stroma | Homogenic | |
Heterogenic | ||
Measurements of ovarian mass (inconsistent in normal ovarian function) | Measurement of the ovarian mass in three perpendicular planes (b1, b2, b3) largest diameter in mm | b1, b2, b3 |
Morphology of mass | Echogenicity of solid component | Heterogenic or homogenic |
Solid: high echogenicity suggesting presence of tissue | Cystic or solid or | |
Cystic-solid | ||
Cystic content | Anechogenic | |
Low level | ||
Ground glass | ||
Hemorrhagic | ||
Mixed | ||
Septum complete/incomplete | Complete | |
Thin strands of tissue running across the cyst cavity | Incomplete | |
An incomplete septum is not completed in some scanning planes | ||
The thickness of thickest septum (S) is measured | Thickness septum (S) | |
Locules | Numbers of locules (L) is counted | L |
Internal wall | Smooth, irregular | |
Any solid projection from the cystic wall ≥3 mm | Solid papillary projections | |
Measurement of largest solid component in three perpendicular planes (height(h), base(ba1), base(ba2)) | Height(h), base(ba1), base(ba2) | |
Cyst type | (No septae and no solid parts) | Unilocular |
No septae, but solid parts | Unilocular solid | |
More than one septae, no solid parts | Multilokular | |
More than one septae, and solid parts | Multilokular solid | |
Not classifiable | ||
Doppler assessment | Color score: (1) no color flow; (2) with minimal color; (3) moderate color; (4) abundant color is presented | Color score 1–4 |
Ascitis | Presence of ascites is noted, largest pouch of fluid in pouch of Douglas is measured (F) in a saggital plane largest diameter (mm) | F |
In endometriosis, visualization of the relationship with the vaginal wall and the rectum may be important to diagnose rectal involvement (Hudelist et al. 2011). To evaluate deep rectovaginal endometriosis, the probe should be placed on the posterior cul de sac of the vagina, and when the probe is slowly withdrawn through the vagina the utero sacral ligament, posterior fornix and the rectovaginal septum can be visualized. By gentle movement of the probe the rectal mucosal and the recto sigmoid wall can be identified and evaluated for deep infiltrating endometriosis. Visualization may be increased by large amounts of gel in the vagina.
1.3.2 Saline Infusion Sonography (SIS) and Gel Infusion Sonography (GIS)
Negative contrast agent such as saline or gel can increase the visualization and outline of abnormalities in the uterine cavity at TVS. Polyps, myomas, synekia, cesarean section scars, and uterine anomalies are often more clearly visualized by contrast in the uterine cavity. When saline is used as contrast agent, it is called saline infusion sonography (SIS), while the use of gel as contrast agent is called gel infusion sonography (GIS).
1.3.2.1 Submucous Myomas for Hysteroscopic Surgery
Submucous myomas for hysteroscopic surgery may at GIS/SIS be evaluated as outlined in Table 1.2 and displayed in Fig. 1.2, and may be scored by the STEPW system to predict complexity of surgery (Lasmar et al. 2011) (Table 1.2). The STEPW system consists of the following five parameters, where a score of 0–2 is given according to Table 1.2:
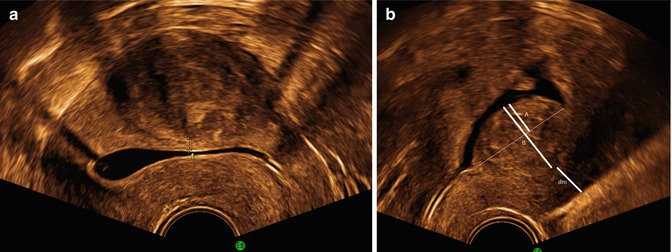

Fig. 1.2
A myoma type 2–5 (a) with impact on the serosa and endometrium (SIS), and (b) a myoma type 2 with measurement of the proportion of the myoma in the uterine cavity (P). B is largest diameter perpendicular to the uterine cavity and A is part of this diameter in the uterine cavity P = 6 A2 (B/2 –A/3)/B3
(a)
Size: Myomas (largest diameter)
(b)
Topography: Defined by the third of the uterine cavity where the myoma is situated (lower, middle and upper third).
(c)
Extension of the base of the myoma: Base of myoma covers (one-third of the wall, one- to two-thirds of the wall, and more than two-thirds of the wall).
(d)
Penetration of the myoma into the myometrium: Type 0,1,2 myoma
(e)
Wall: when the fibroid is on the lateral wall, one extra point is added.
The total sum score predicts complexity of hysteroscopic surgery.
1.3.3 Three-Dimensional Transvaginal Ultrasound (3D-TVS)
3D-TVS is just a collection of 2D images added together in a volume, which allow reconstruction and evaluation of the scan in different planes. Resolution at 3D will never be better, than at the original 2D images. In the presence of poor image quality at 2D, a collection of images of poor quality at 3D will seldom be helpful. Most important feature at 3D–TVS is the ability to “reconstruct” the uterus to provide a coronal view.
Procedure: For 3D-TVS of the uterus, volumes are obtained at a mid-sagittal view of the uterus. The image settings at 2D should be optimized, and magnified to an optimal image with a minimum amount of free space around the region of interest (uterus). The volume box for sampling of the 3D images should be applied again with a limited amount of free space around the uterus. Both the patient and the probe should not be moved during collection of the volumes.
To obtain a reconstruction of the coronal plane, the volume of the uterus should be perfectly aligned along the mid long axis in the sagittal plane and along the axis of the uterine horns in the transverse planes. This can be achieved by the Z-technique (Abuhamad et al. 2006), which include three steps (Fig. 1.3).
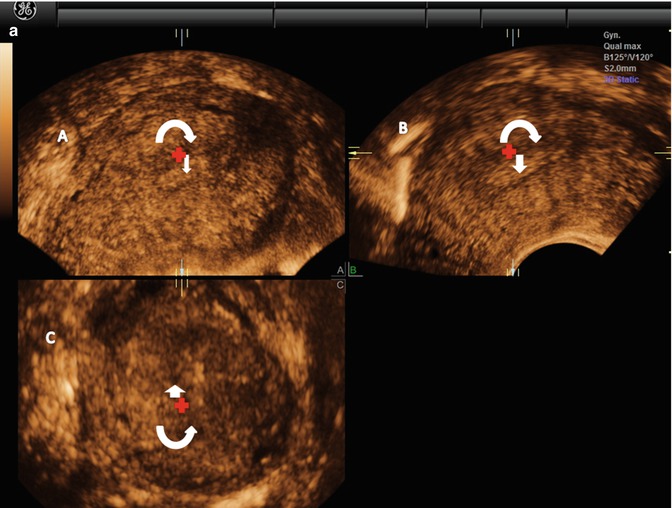
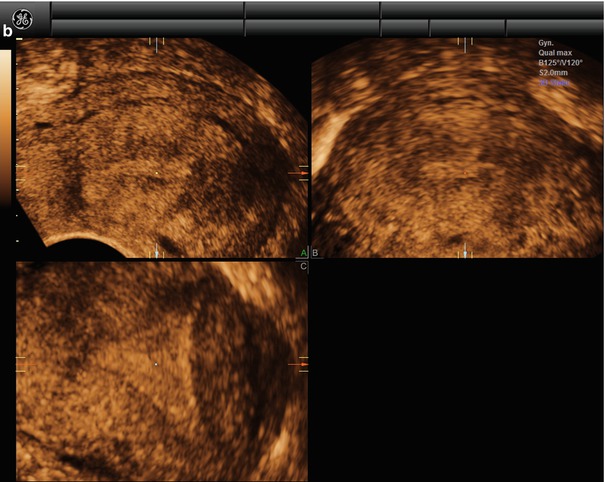
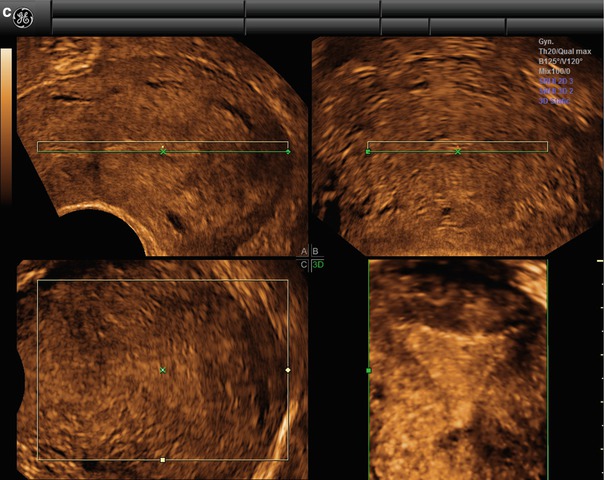



Fig. 1.3
(a) Step A. The reference/rotational point (+) is placed in the midlevel of the endometrial stripe in the sagittal plane. Z rotation is used to align the long axis of the endometrial stripe along the horizontal axis in the sagittal plane of the uterus. Step B. The reference/rotational point (+) is placed in the midlevel of the endometrial stripe in the transverse plane. The Z rotation is used to align the endometrial stripe with the horizontal axis in the transverse plane of the uterus Step C. Z rotation is applied on plane C to display the mid coronal plane in the traditional orientation in plane C. (b) In the C plane is the coronal view displayed (VCI-settings). (c) Postprocessor rendering is performed
The time spent on producing a perfect coronal view is less than 1 min after limited training (Abuhamad et al. 2006). A perfect coronal view and a simple scroll through the images in the C-plan may in clinical practice supply most additional information given by 3D-TVS.
There is a substantial learning curve and time involved to manage all the different features in post-processing, which limits the common use in clinical practice. However, two or three features can easily be learned and may in most clinical situations cover the need.
At SIS a 3D volume of the uterus can be obtained (3D-SIS), which allow reconstructing the outline of the uterine cavity. However, an optimal reconstruction without acoustic shadows from an intrauterine catheter is achieved with gel in the uterine cavity, and the catheter should be removed before volume sampling (3D-GIS). 3D-GIS may be helpful in some clinical situations especially in indefinite coronal view at 3D-TVS (Mavrelos et al. 2011; Caliskan et al. 2010; Makris et al. 2007; de Kroon et al. 2004).
1.3.4 Three-Dimensional Power Doppler Angiography (3D-PDA)
A three-dimensional power Doppler angiography (3D-PDA) can be performed to study the distribution, pattern, and vascular branching of the vascular vessels. Power Doppler is simply applied to the volume box and a 3D volume is obtained. The technique also allow for a more objective reproducible (Raine-Fenning et al. 2003) assessment of uterine vascularization, although the measurement is dependent on machine settings (Raine-Fenning et al. 2008).
1.3.5 Dynamic Contrast-Enhanced MRI (DCE MRI) and Diffusion-Weighted MRI (DW MRI)
Tissue perfusion in pelvic masses and uterine masses can be evaluated by DCE MRI (Nakai et al. 2008; Paldino and Barboriak 2009). In tissue with high perfusion there will be high signal intensity in T2-weighted images after injection of contrast, while tissue without perfusion will have low intensity at T2-weighted images. Tissue perfusion can be assessed in two ways: a semi-quantitative method (dependent on individual system and patients) which analyze the changes in signal intensity, and a quantitative method using a pharmacokinetic system and patient-independent model.
Diffusion-weighted (DW) MRI is based on diffusion motion of water molecules. Signals are dependent on microscopic water diffusivity, and decrease in the presence of factors that restrict water diffusion, such as cell membranes and the viscosity of the fluid. Signals are influenced by changes in the balance between extracellular and intracellular water molecules, and changes in cytologic morphology including the nuclear-to-cytoplasm ratio and cellular density. The technique can easily be added to any routine MR protocol.
1.3.6 Contrast Enhanced Ultrasound (CEUS)
CEUS is an alternative upcoming method with use in other specialties (Piscaglia et al. 2012), to evaluate perfusion after different treatment modalities. CEUS has been used when HIFU has been monitored by TVS, and seems to be a promising low cost method (Zhou et al. 2007). Enhancement in the tissue is observed after contrast injection supported with in-built or off-line software to measure the degree of enhancement. The time of enhancement and the distribution is observed.
1.4 Diagnosis of Uterine Pathology
1.4.1 Abnormal Uterine Bleeding
The newly established FIGO system – the PALM-COIN ((P) polyp, (A) adenomyosis, (L) leiomyoma, (M) malignancy/hyperplasia-(C) Coagulopathy, (O) Ovulatory dysfunction, (I) Iatrogenic, and (N) Not classified) system – has been elaborated (Munro et al. 2011).
A patient with abnormal uterine bleeding should be categorized according to this system. The PALM – part of the system – is mainly based on ultrasound and pathologic evaluation of the endometrium. The COIN – part of the system – is evaluated by the patient history and laboratory analysis.
1.4.2 Endometrial Pathology
1.4.2.1 Premenopausal Bleeding
TVS, GIS, or SIS are very efficient methods to rule out pathology in the uterine cavity as polyps and myomas in premenopausal bleeding. To rule out polyps and myomas, TVS seems to miss one in five endometrial polyps (de Kroon et al. 2003; Dueholm et al. 2001a, b), while SIS is in line with hysteroscopy (HY). TVS is able to identify myomas, but a differentiation between myomas of type 1–3 most often require GIS/SIS, which is important for selection of myomas for either laparoscopic or hysteroscopic treatment (Dueholm et al. 2002a). Patients with symptomatic focal pathology will have a time-efficient planning of HY by TVS supplied with SIS or GIS. This will provide clear information of the size, number and type of pathology in the uterine cavity, and thereby patients can be selected for outpatient mini-hysteroscopy at an office setting, two-generation endometrial ablation, inpatients resectoscopic surgery, and also to surgeons with the required experience. Small intrauterine pathology with diameter below 2 cm (Bettocchi et al. 2004; Cicinelli 2010) is removed at outpatient mini-hysteroscopy. The complexity of resectoscopic hysteroscopic myoma surgery can be predicted by the findings at SIS/GIS and scored by the STEPW system (Table 1.2). Complex surgery (score five or more) should be performed by experienced surgeons, and a two-step procedure and preoperative medical treatment should be considered. UAE or laparoscopic myomectomy should be taken into account at scores of more than 7–9.
1.4.2.2 Postmenopausal Bleeding
TVS is the first investigation in postmenopausal bleeding. The endometrium should be investigated and described according to Table 1.1. Figure 1.4 shows examples of heterogenic (a) and cystic endometrium (b). In the presence of a sharp well-defined midline echo with endometrial thickness of ≤3 or ≤4 mm only 2 % respective 5 % of endometrial cancers will be missed (Timmermans et al. 2010). At an endometrial thickness >3–4 mm endometrial sampling is performed (Timmermans et al. 2010).
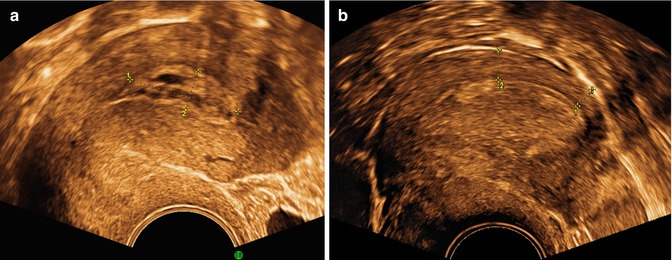

Fig. 1.4




A cystic polyp is seen in (a) with cystic regular endometrium, clear margins and bright line. A single small vessel was seen at Doppler. In (b) endometrial cancer, with a thickened heterogenic endometrium with regular endomyometrial junction at the posterior wall, (marked), but irregular not defined margins at the anterior wall
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


