Introduction
The extracorporeal fertilization of mammalian oocytes was first reported over three decades ago by the British team of Steptoe and Edwards. They were the first to report a pregnancy after in vitro fertilization (IVF) of a human egg and also the first to achieve the birth of an in vitro fertilized baby. Since then, hundreds of thousands of pregnancies have been achieved worldwide by IVF and its modifications, collectively known as assisted reproductive techniques (ARTs). As experience has accumulated, success rates have increased and the indications for the ARTs have expanded. According to the 2006 CDC report, 138,198 ART cycles took place in the United States during that year, resulting in 41,343 live births, and 54,656 infants, representing slightly more than 1% of all US births.
In vitro fertilization essentially performs the function of the fallopian tube in that it brings together the oocyte and sperm, allows for fertilization and then delivers the resulting embryo to the uterine cavity. Therefore, the initial experience with IVF involved women with tubal disease that could not be surgically corrected. With its efficacy established, IVF was progressively made available to patients with other infertility diagnoses. IVF is utilized when conventional therapy fails or when there is little hope that other therapy will be successful. Current indications for IVF include:
- absent or blocked fallopian tubes
- failed tuboplasty
- endometriosis
- male factor infertility
- unexplained infertility
In counseling patients with open fallopian tubes and without a severe male factor, alternative treatment options, including observation, must be considered, as some groups of IVF candidates have substantial treatment-independent pregnancy rates. The difficulty in deciding how soon to proceed to IVF arises in women of advanced reproductive age, in whom further expectant management may risk the occurrence of ovarian failure. In general, women with nontubal factor infertility are offered between three and six cycles of superovulation and intrauterine insemination (IUI) prior to proceeding to IVF. Among younger women, waiting for a total of 2 years of unprotected intercourse and/or conventional treatment is a reasonable guideline which may be used in counseling potential candidates.
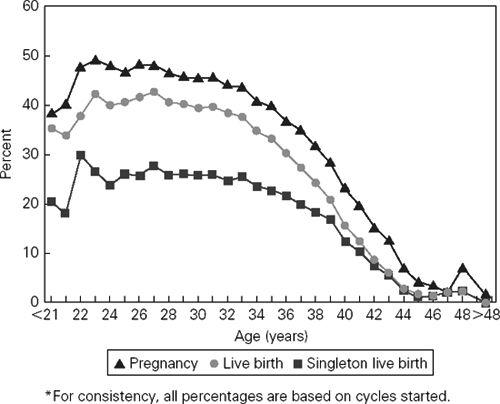
One of the principal determinants of IVF success is the age of the female partner. Women over the age of 40 years experience a substantially reduced likelihood of pregnancy (Figure 103.1). This decrease in success parallels that observed with other forms of fertility treatment. In IVF, the diminished success is due both to a decreased response to gonadotropin stimulation, resulting in a decreased number of embryos available for transfer, as well as decreased per embryo implantation rates. Additionally, pregnancies in older women are associated with increased miscarriage rates.
Figure 103.1 Percentage of ART cycles using fresh nondonor eggs or embryos that resulted in pregnancies, livebirths and singleton livebirths, by age of woman. Reproduced from Center for Disease Control and Prevention: www.cdc.gov.
In addition to the advanced chronologic age of the female partner, elevated follicle-stimulating hormone (FSH) levels in the early follicular phase are associated with poor prognosis for pregnancies with IVF. A single FSH measurement may not be sufficient, as serum FSH levels are known to fluctuate from cycle to cycle. In one study addressing this issue, patients with one prior elevated (>20 mIU/mL) FSH level but normal subsequent levels were observed to achieve a 5.6% pregnancy rate. Patients with two or more previous elevated FSH levels did not achieve any pregnancies.
Recent data suggest that in addition to elevated early follicular phase FSH levels, elevated estradiol (E2) levels are also associated with a poor pregnancy outcome with IVF. One report identified a level of 75 pg/mL as being discriminatory between cycles with good and those with poor prognosis. We consider it reasonable to obtain day 3 serum measurements of E2 and FSH in all women over the age of 35 who are contemplating IVF, and to cancel cycles in those with FSH levels greater than 20 mIU/mL and/or E2 levels greater than 100 pg/mL. If levels are elevated upon repeated measurements, other forms of therapy (e.g. oocyte donation) should be considered. Younger patients who exhibit a poor ovarian response to exogenous gonadotropins should also be tested.
The follicular phase: superovulation
The human ovary normally produces only one dominant follicle and, thus, one mature oocyte. However, if additional stimulation with FSH is provided, the ovary will respond by producing multiple follicles. ART cycles generally utilize some form of ovarian hyperstimulation in order to increase the number of oocytes and subsequently embryos available for transfer, as each embryo has a chance of implanting and thus, pregnancy rates correlate best with the number of embryos replaced.
The most commonly utilized stimulation regimen consists of gonadotropin-releasing hormone agonist (GnRH-a) which suppresses endogenous gonadotropin production (thus maximizing cycle control) and subsequent ovarian stimulation with exogenous gonadotropins. The GnRH-a is initially given for about 2 weeks until pituitary downregulation is achieved. It is then continued during the stimulation phase in order to prevent a premature luteinizing hormone (LH) surge. The dose of FSH is usually started at 225–300 IU/day given intramuscularly. Patients with polycystic ovarian disease and those who have previously demonstrated an exaggerated response may be given a lower initial dose (75–150 IU/day). The dose is subsequently adjusted according to follicular growth and serum levels of E2.
Monitoring of the ovarian response to stimulation is achieved by transvaginal ultrasonographic measurement of the size of the developing follicles and the measurement of serum E2 levels. Ovulation is triggered with human chorionic gonadotropin (hCG) 10,000 IU intramuscularly when follicle maturity is obtained. Maturity criteria are based on both follicle size and serum E2 levels.
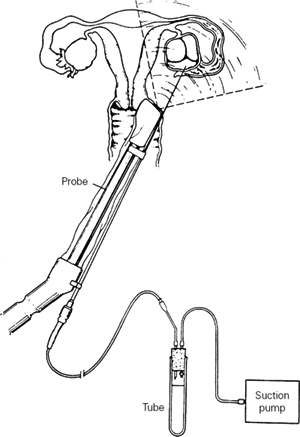
Transvaginal ultrasound-guided follicle aspiration is the most commonly used method of oocyte retrieval. It is performed 34–36 hours after hCG administration with analgesia achieved by conscious sedation. Under direct ultrasonographic visualization, the aspirating needle is introduced sequentially into each follicle and the follicular fluid aspirated (Figure 103.2). The reported incidence of complications of transvaginal follicle aspiration is low; only one series found a substantial risk of infection and the reported 3% incidence of infection dropped to 0% with the use of prophylactic antibiotics. The removal of hydrosalpinges prior to IVF (see below) reduces the risk of infection associated with this procedure.
Figure 103.2 Transvaginal ultrasound-guided follicle aspiration. The ultrasound probe is covered with a sterile plastic sheath.
Oocytes are separated from the follicular fluid in the laboratory under a dissecting microscope. The size of the oocytes (approximately 70–100 μm diameter) allows their visualization and mechanical transport with a pipette to a wash dish, and subsequently a holding test tube containing culture medium. When all the oocytes have been identified and isolated, they are transferred to the incubator where they are kept until insemination approximately 6 hours later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


