95 Human Immunodeficiency Virus
Diagnosis
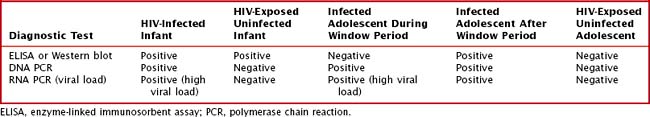
Table 95-1 summarizes the expected results of HIV diagnostic tests for HIV-exposed and -infected infants and adolescents. Children older than the age of 18 months would have the same test results as adolescents in the same infection or exposure category.
Clinical Manifestations of Hiv Infection
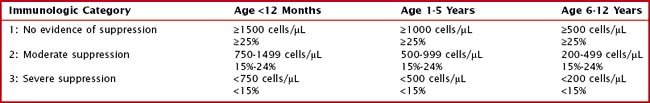
The process of HIV replication leads to depletion of CD4+ T lymphocytes. The degree of immunologic suppression is classified based on the number and percent of CD4+ T lymphocytes present in the bloodstream. In young children, the normal number of CD4+ T lymphocytes is much higher than in adults. Therefore, age-specific absolute CD4+ T lymphocyte count ranges should be used to determine the degree of immune suppression in children. CD4+ T lymphocyte percents change less with age and can be used instead of absolute counts to classify the degree of immune suppression in HIV-infected children (Table 95-2).
Table 95-2 Immunologic Categorization Based on Age-Specific CD4+ T-Lymphocyte Counts and Percent of Total Lymphocytes
Along with depletion of CD4+ T lymphocytes, HIV infection leads to functional defects in existing CD4+ T-lymphocytes and defects in B-cell function. These combined immunosuppressive processes lead to a number of clinical manifestations. The most severe and common of these manifestations are outlined in Table 95-3. Opportunistic infections, cancers, hematologic aberrations, and other noninfectious manifestations are among the most severe AIDS-defining conditions.
Table 95-3 Selected Clinical Manifestations of HIV Infection
| Severe Manifestations | Description |
|---|---|
| Pneumocystis jiroveci pneumonia | Definitive diagnosis via microscopy of induced sputum or BAL |
| Multiple or recurrent serious bacterial infections | Septicemia, pneumonia, meningitis, bone or joint infection, internal organ infections |
| Kaposi’s sarcoma | Characterized by pink or purple lesions on the skin and soft tissues; diagnosis is confirmed with biopsy |
| Lymphoma | Cerebral or B-cell non-Hodgkin’s lymphoma |
| Mycobacterial infections | Extrapulmonary mycobacterium tuberculosis infection and nontuberculous mycobacterial infections |
| HIV encephalopathy | Failure to attain or loss of developmental milestones or loss of intellectual ability, impaired brain growth, or acquired symmetric motor deficits lasting for >2 mo without a cause other than HIV |
| HIV wasting syndrome | Unexplained severe wasting, stunting, or severe malnutrition not adequately responding to standard therapy |
| Severe herpes simplex infections | Bronchitis, pneumonitis, esophagitis (or mucocutaneous ulcer persisting >1 mo) |
| Severe candidiasis | Esophageal or pulmonary (including bronchi and trachea) |
| Moderately Severe Manifestations | |
| Single episode of serious bacterial infection | Septicemia, pneumonia, meningitis, bone or joint infection, internal organ infections |
| Lymphoid interstitial pneumonitis | Definitive diagnosis via biopsy but characterized by chronic bilateral reticulonodular interstitial pulmonary infiltrates and hypoxemia |
| Recurrent or chronic diarrhea | Persistent ≥14 days |
| Anemia, neutropenia, or thrombocytopenia persisting ≥30 days | Anemia: hemoglobin <8 g/dL Neutropenia: ANC <1000 cells/mm3 Thrombocytopenia: platelets <100,000 cells/mm3 |
| Herpes zoster | At least two distinct episodes or more than one dermatome |
| Herpes simplex virus | Recurrent stomatitis (>two episodes in 1 year) |
| Complicated varicella | Disseminated or severe chicken pox |
| Candidiasis | Oropharyngeal lasting for >2 mo |
| Mild Manifestations | |
| Lymphadenopathy | ≥0.5 cm at more than two sites |
| Recurrent or persistent upper respiratory tract infections | Including sinusitis or otitis media |
| Hepatosplenomegaly | Unexplained, persistent |
| Mucocutaneous lesions | Extensive wart virus infection, extensive molluscum contagiosum, popular pruritic eruptions, recurrent oral ulcers |
ANC, absolute neutrophil count; BAL, bronchoalveolar lavage.