Hirsutism is thick, dark terminal hair on androgen-sensitive areas of the body, such as the chin and upper lip (a), pelvis and linea alba (b), and sideburns and neck (c).
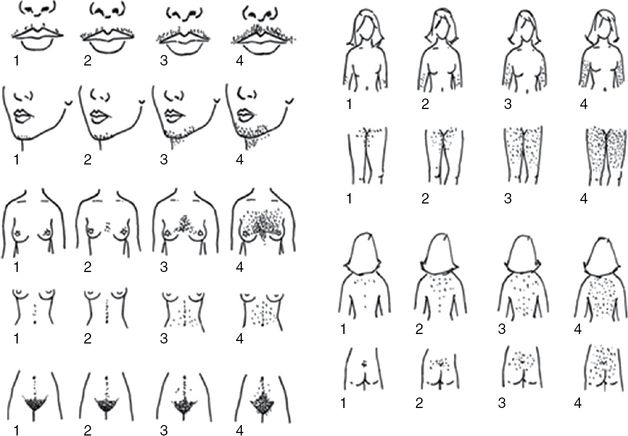
Ferriman–Gallwey hirsutism scoring system. Each of the nine body areas most sensitive to androgens is assigned a score from 0 (no hair) to 4 (frankly virile), and these separate scores are summed to provide a hormonal hirsutism score. (Reproduced from Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society Clinical Practice Guideline) [11].
Many factors must be considered during the evaluation of hirsutism, including ethnicity, age, obesity, and genetics. There is a great amount of racial variation in hair growth and distribution, which is largely a reflection of varying 5α-reductase activity levels at the hair follicle. Black and white women have similar rates of hirsutism. East Asian women tend to have lower rates, while Southeast Asian women generally have higher rates [3]. As previously stated, hyperandrogenism decreases with age, which may affect phenotype. It is important to evaluate body hair in preadolescents, as pubic hair before age 8 years may be an ominous indication of future PCOS [12]. Further, hirsutism rates seem to correlate directly with body mass index (BMI), and may be a result of increased androgen levels in obesity [13]. Likewise, insulin resistance may increase hirsutism [14]. Family history is also important as mothers and sisters of PCOS patients are often hirsute [15]. Additionally, some hairiness may be genetic. Finally, it is imperative to ask about hirsutism even if there is little evidence on the physical exam as the patient may be controlling hair growth by shaving, waxing, electrolysis, or other cosmetic procedures.
Acne
Both acne vulgaris and alopecia are less common physical manifestations of hyperandrogenism, but if present are still useful to the clinical picture of PCOS. Androgens influence acne by stimulating sebum production, which allows Propionibacterium acnes to colonize the follicle; inflammation results. The prevalence of acne in PCOS is similar to the normal population. While some studies have suggested it has a higher prevalence and is a useful finding, the AES determined the prevalence to be 15–25%, which is similar to the normal population [3]. Part of the difficulty is in the assessment. Although systems have been created to grade acne severity, none have been validated as standard. When assessing acne, it may be best to give a global assessment – mild, moderate, or severe – based on the type of lesions (papules, pustules, nodules, cysts) and extent of skin involvement. It is also important to ask about duration of acne and prior treatments. Recalcitrant acne that persists into a woman’s late teens or early twenties may be suggestive of a hyperandrogenic etiology [9].
Race, medications, and obesity may affect the presentation of acne. Like hirsutism, the prevalence of acne also varies by race. It is more commonly observed in Asian Indians and less commonly in Pacific Islanders [3]. Topical and oral acne medications can mask the clinical presentation of acne and their use should be evaluated in the history. Obese PCOS women often have less acne, although the reason is unclear [8]. Overall, acne seems to be less helpful in establishing a diagnosis of hyperandrogenism.
Alopecia
Androgenic alopecia, or male pattern balding, reflects endogenous androgen activity on the pilosebaceous unit. It typically begins at the vertex, spreads to involve the entire crown, and eventually leads to diffuse hair loss [9]. Its prevalence in PCOS is variable depending on the study, and there is generally poor correlation between biochemical hyperandrogenism and alopecia [3]. This finding is likely explained by varying sensitivities of the pilosebaceous unit to circulating androgens. Alopecia is relatively underdiagnosed, which may be because hair loss is typically noticed later than hair gain [16]. Generally, a woman becomes aware of thinning hair on her scalp only after a 25% reduction. On the one hand, the presence of alopecia is a strong indicator of PCOS; PCOS may account for 10–40% of all women with alopecia [3]. However, PCOS rarely presents with alopecia. Further, alopecia has low specificity for PCOS, since it has many other causes including genetic, environmental, and nutritional. In general, it seems to be more effective to evaluate hirsutism, since alopecia is rarely present independently from hirsutism [17].
Acanthosis nigricans and skin tags
A key physical exam finding of insulin resistance in PCOS is acanthosis nigricans. The lesion is characterized by thick, velvety, hyperpigmented plaques on the intertriginous surfaces, predominantly the neck and axilla. This is depicted in Figure 16.3. It is often asymptomatic, but may be occasionally pruritic. Its prevalence in PCOS is 15% alone, but 65% with obesity [18]. In fact, it appears to be relatively dependent on weight, and may regress with weight loss. It is important to note the physical exam has a low sensitivity for detecting acanthosis nigricans. Further, acanthosis nigricans is a fairly nonspecific finding as it is common to all conditions with insulin resistance including type 2 diabetes (DM) and Cushing’s syndrome.
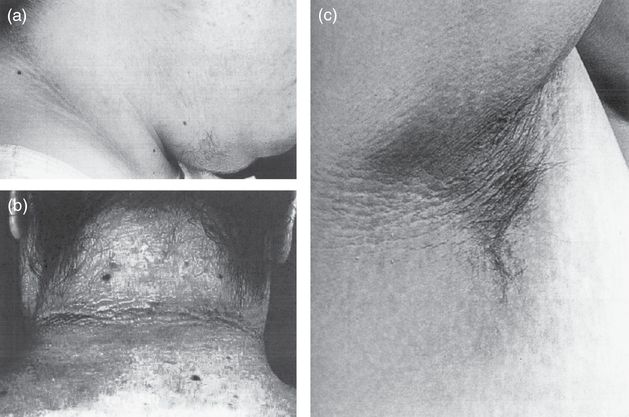
Acanthosis nigricans is characterized by thick, velvety, hyperpigmented plaques on the intertriginous surfaces, predominantly the neck (a, b) and axilla (c). Note also the terminal hair growth on the chin in (a).
Acrochordons, or skin tags, are small, soft, flesh-colored, pedunculated, benign neoplasms. Their growth is linked to insulin resistance and they can often be found in and around the lesions of acanthosis nigricans. Their presence is also rather nonspecific.
Ovulatory dysfunction
Ovulatory dysfunction is a cornerstone feature of PCOS and is identifiable from a history of menstrual features and timing. PCOS is the most common cause of ovulatory dysfunction [6]. The root of ovulatory dysfunction in PCOS is decreased ovulation. The level of anovulation in each patient determines her menstrual characteristics. The PCOS phenotype ranges from no menstruation (amenorrhea) to limited menstruation (oligomenorrhea) to normal cycles (eumenorrhea) and even, in rare cases, increased menstruation (polymenorrhea). The average menstrual interval is 28 days with a range of 21–35 days. Oligomenorrhea is menstrual bleeding occurring at equal to or greater than a 35-day interval or fewer than 8 times per year. Polymennorhea is menstrual bleeding occurring more frequently than every 21 days. A history of irregular bleeding without the typical premenstrual symptoms – bloating, mood changes, and breast fullness – is suggestive of anovulation.
Anovulatory cycles in PCOS can present immediately after menstruation or at a later time [18]. The prevalence of anovulation in PCOS is high (75–85%) and four times more prevalent than the regular population [3]. Amenorrhea in PCOS is present in 16%, while oligomenorrhea is present in 68%. [18] Eumenorrhea is present in 20–30% of women with PCOS, although these women often have biochemical evidence of ovulatory dysfunction [3]. Thus a history of regular menstruation does not exclude the diagnosis. An absence of premenstrual symptoms in a eumenorrheic-reporting woman may indicate anovulation. In this case, a midluteal serum progesterone may be a useful consideration [6]. Less than 2% of PCOS patients are estimated to have polymenorrhea [3]. Patients with a more severe PCOS phenotype have more infertility and higher androgen levels.
Several criteria must be considered in evaluating ovulatory dysfunction. PCOS females may have transitory menstrual regularity over time; they may initially have regular cycles, and then develop irregularity. However, most patients state that their cycles never established regularity after menarche. Adolescents with persistent oligomenorrhea or amenorrhea for more than 2 years after menarche should be evaluated for PCOS [6]. Cycle regularity can also return to PCOS patients, as was shown in a 4- to 7-year follow-up [19]. An accurate history including frequency and duration is critical, as PCOS females may incorrectly believe they have a normal menstrual interval, not oligomenorrhea. Age has a strong impact on menstrual cycles. Early adrenarche and early breast development is associated with PCOS [20]. Menstrual dysfunction commonly decreases closer to menopause [21]. Anovulation in a woman’s twenties can disappear by her forties, while ovulatory cycles increase [22]. Additionally, later menopause onset may be associated with PCOS [23]. Thus, the patient’s age at thelarche, adrenarche, and menarche should be documented in the history [9]. Higher body weight also commonly worsens ovulatory dysfunction [24]. Drug history must be considered as oral contraceptives can mask ovulatory dysfunction.
A thorough family history of ovulatory dysfunction is important for complete evaluation. Mothers of PCOS patients had increased menstrual dysfunction [15]. Thirty percent of the mothers of PCOS patients also had amenorrhea [25]. Finally, it is also very important to realize that it takes time to establish an individual’s menstrual cycle pattern. A provider must determine what is normal for each patient before making the diagnosis.
The differential diagnosis for menstrual dysfunction can be elucidated by the history. Lifestyle changes, such as the start of intense exercise or a rapid weight change, can both lead to transitory menstrual irregularity. Patients should be questioned about eating disorders, including anorexia and bulimia. Further testing will be needed to sort through other potential causes such as pregnancy, premature ovarian failure, hyperprolactinemia, outflow tract obstruction, thyroid dysfunction, and hypothalamic amenorrhea.
Metabolic syndrome
PCOS patients seem to be at a moderately increased risk of developing metabolic syndrome. Metabolic syndrome is defined as a syndrome that predisposes a person to increased risk of type 2 DM and coronary artery disease (CAD). There are multiple definitions of the specific features of the syndrome, which can complicate research. Loosely, metabolic syndrome is a constellation of central obesity, high fasting glucose, hypertension, and dyslipidemia. The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) definition for metabolic syndrome is one of the most widely used [26]. It was later updated by the American Heart Association and the National Heart Lung and Blood Institute [27]. The NCEP ATP III definition is presented in Table 16.3.
Risk factor | Criteria |
|---|---|
Abdominal obesity (defined by waist circumference) | >88 cm (>35 in) |
Triglycerides | ≥150 mg/dL |
HDL cholesterol | <50 mg/dL |
Blood pressure | ≥130/≥85 mmHg |
Fasting glucose | ≥100 mg/dL |
Metabolic syndrome in PCOS can be evaluated in the initial history and physical exam [6]. BMI and waist circumference should be measured initially and monitored over time. Insulin resistance must be evaluated through patient history as well as physical exam by careful screening for acanthosis nigricans. Blood pressure can be measured in office, and dyslipidemia can be evaluated in subsequent laboratory testing.
Several studies support PCOS as a metabolic syndrome risk, independent of BMI or age. A meta-analysis found PCOS patients to be at increased risk for metabolic syndrome, with and without BMI controlled [28]. Metabolic syndrome was found at a variable prevalence in PCOS patients in recent studies: around 25–35% of PCOS patients compared with approximately 10% in the regular population [29]. The definition used to define PCOS – NIH, Rotterdam, or AES – may affect the prevalence values.
The link between PCOS and metabolic syndrome may be affected by many factors, including BMI, race, and lifestyle. The BMI of PCOS patients can help determine the prevalence of metabolic syndrome [30]. For physical exam purposes, abdominal obesity seems to be the most critical PCOS risk factor to evaluate for metabolic syndrome and insulin resistance [31]. Race, not PCOS, may predispose certain patients to metabolic syndrome. South Asian PCOS patients have higher metabolic syndrome prevalence than Caucasian patients [32]. Culture may also play a large role, as shown by a study comparing Chinese women living in China to American Chinese women [33]. It was concluded that mainland Chinese women with PCOS had a lower risk of metabolic syndrome than Chinese women in Westernized societies. Similarly, diet may be a factor.
Metabolic syndrome screening should be performed in every patient suspected of PCOS. Acanthosis nigricans of insulin resistance, obesity, and hypertension can be easily evaluated in the physician office. Lipid screening should also be done as obese PCOS patients are at risk for dyslipidemia, especially with accompanying hyperandrogenism [34]. As complications of metabolic syndrome, DM and CAD should be fully evaluated in each PCOS patient.
Obesity
Though not part of the common PCOS definitions, obesity is highly prevalent in PCOS patients. It is responsible for excessive morbidity and should be evaluated during the physical exam with BMI calculation and measurement of waist circumference, using the National Health and Nutrition Examination Survey method [6,35]. With the patient standing upright, the waist circumference is measured using a nonfolded measuring tape positioned at the top of the iliac crest and parallel to the floor. The measurement is then taken at the end of the patient’s expiration. A waist circumference of greater than 88 cm is considered abnormal in Caucasian American and African American women and greater than 80 cm in Hispanic, Native American, Asian, and European women [35]. The prevalence of obesity in PCOS patients is between 30% to 70%, 50% even in adolescents, and appears across cultures and races [29,24,36].
The relationship between obesity and PCOS is not clearly defined. Some evidence indicates that PCOS is more prevalent in the severely obese, suggesting obesity may influence PCOS development [37]. Others argue that the obesity in PCOS is a greater reflection of environmental factors, noting a parallel increase in the BMI of PCOS patients with the prevalence of obesity in a surrounding population over a 15-year period [38]. Regardless, obesity in PCOS patients causes a multitude of other effects. Obesity increases the severity of other PCOS features such as androgen levels, hirsutism, ovulatory dysfunction, infertility, and pregnancy complications. Interestingly, it tends to ameliorate acne. The influence of obesity on the features of PCOS is likely due to a worsened underlying relative hyperandrogenism [6]. This results when high levels of insulin suppress the hepatic production of sex hormone-binding globulin (SHBG), leading to increased bioavailable androgens.
The presentation of obesity in PCOS patients can occur in childhood before the cornerstone PCOS features of hyperandrogenism and ovulatory dysfunction develop, or it may occur in adulthood. If obesity is present at the time of evaluation, the patient should be questioned about its onset and progression [9]. Body fat distribution can be easily evaluated in the PCOS physical exam. Abdominal fat, specifically, is associated with PCOS obesity. Patients with abdominal obesity, not peripheral, have worse hyperandrogenemia and are at increased risk for developing metabolic syndrome [39,40].
Race, family history, culture, age, and diet should be considered in obesity evaluation in PCOS. Black and Hispanic PCOS women are more likely to be obese than Asian and white PCOS women [41]. Male relatives of PCOS patients are more obese and thus, more prone to metabolic syndrome [42]. First-degree relatives of a PCOS patient have higher body fat levels than the unrelated population [43]. Culture also seems to affect obesity development in PCOS patients. American women with PCOS are more likely to be obese than those from other countries, suggesting a lifestyle effect [3]. Older, postmenopausal PCOS patients also have higher obesity rates [44]. Patient eating habits must also be accounted for. A poor diet may worsen other PCOS features, like insulin resistance; a healthy diet should always be encouraged.
Insulin resistance and glucose dysregulation
Insulin resistance is a metabolic syndrome feature that should be evaluated in the history of any suspected PCOS patient. Patients may complain of lethargy, constant hunger, and difficulty concentrating, which are all nonspecific. Acanthosis nigricans is a clinical manifestation that can be evaluated in the physical exam and is the direct result of a compensatory hyperinsulinemia. In fact, some hypothesize that insulin resistance and the subsequent hyperinsulinemia largely underpin the formation of PCOS in many women. Hyperinsulinemia drives ovarian androgen production and suppresses hepatic SHBG production. The result is increased circulating free androgens. The capacity of otherwise healthy, young women to produce high levels of insulin may mask overt diabetes measured by fasting blood glucose. For this reason, each suspected patient should be screened with a 2-hour 75 g oral glucose tolerance test (OGTT) measuring both insulin and glucose. PCOS patients are at risk for insulin resistance and the prevalence of insulin resistance in PCOS is around 50–70% [3].
Insulin resistance in PCOS can be modulated by many factors. Obesity tends to increase insulin resistance [45]. Likewise, insulin resistance may occur in up to 95% of obese women with PCOS [35]. Insulin resistance worsens with age, while other portions of the disorder may improve [7]. Anovulatory PCOS patients also have higher and more variable insulin resistance than ovulatory patients [46]. Diet also seems to affect insulin resistance in PCOS patients, although the effect may be small [38]. A family history of insulin resistance or type 2 DM can also be supportive as first-degree relatives of PCOS patients have more insulin resistance, and insulin resistance is also more common in individuals with a family history of type 2 DM [36, 43].
PCOS is associated with a 5- to 10-fold increase in the risk of type 2 DM, which should be suspected in any female with the disease. The prevalence of type 2 DM amongst American PCOS patients is 3–10%, and that of glucose intolerance is 30–35% [6]. Over time, many PCOS patients progress from glucose intolerance to type 2 DM, which underscores the importance of continued monitoring [47]. Symptoms that may clue to the onset of diabetes are excessive thirst, frequent urination, polyphagia, and weight loss. Many patients, though, will be detected only on routine screening. The disease should be checked initially using an OGTT, or hemoglobin A1c if the patient is unable to complete an OGTT [6]. Patients should be rescreened every 3–5 years, or more frequently if there is central obesity, substantial weight gain, or clinical symptoms of diabetes [6]. Factors that may predispose a patient with PCOS to type 2 DM development should also be noted, specifically abdominal obesity and a family history of type 2 DM.
Hypertension
The association between PCOS and hypertension is contentious, but blood pressure should be evaluated as part of the complete PCOS physical exam. Ideally, blood pressure should be 120 mmHg systolic and 80 mmHg diastolic or lower. Hypertension, and prehypertension, should be treated to reduce cardiovascular disease. Studies have shown hypertension to be associated with PCOS, even with obesity and insulin resistance controlled, while others have linked the association to particular subgroups such as women aged 35–55 [48,49]. Other research has indicated a hypertension risk only with obesity and high BMI [50,51]. A family history of hypertension may be important to a PCOS workup, as relatives of a woman with PCOS are more likely to have hypertension [15]. Race of the patient should also be considered; black women with PCOS have a higher prevalence of hypertension when corrected for BMI [41].
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is characterized by episodes of pharyngeal obstruction during sleep and may manifest as daytime somnolence. Generally, sleep disordered breathing is seen in older, obese males and the prevalence amongst premenopausal women is less than 1%. However, amongst women with PCOS, the prevalence increases 30- to 40-fold [52]. The link between PCOS and OSA has not been well elucidated, but likely results from the interplay between hyperandrogenism, insulin resistance, and obesity. OSA in PCOS tends to develop after adolescence, later in the course of the disease [53].
Patients should be carefully questioned about sleep disturbances, as OSA is a risk factor for cardiovascular mortality and morbidity and its treatment may help stave off further metabolic sequelae in PCOS. Patients with OSA may mention a history of snoring, night restlessness, morning headache, and subsequent daytime sleepiness. Their partner may be able to attest to interrupted breathing. OSA is difficult to diagnose on history and physical exam, so patients with a suspicious history should be referred to a certified sleep laboratory for polysomnography [6].
Cardiovascular risk
PCOS patients have cardiovascular disease (CVD) risk factors, such as hypertension, obesity, insulin resistance, and type 2 DM. PCOS patients also have increased levels of CVD risk markers [54]. Meta-analysis indicates that PCOS patients are twice as likely to have a myocardial infarction (MI) or stroke as the regular population and 1.5 times as likely when corrected for obesity [55]. Carotid intima thickness and electron beam studies both show increased disease in PCOS patients [56,57]. However, strong evidence also exists against PCOS patients being at increased risk of a cardiovascular attack, aside from PCOS features. A Mayo Clinic population-based study showed no increase in cardiovascular events, with obesity being the only increased CVD risk factor over controls [58].
In all, there is insufficient evidence to determine the rate of cardiovascular disease or its onset. Therefore, attention has turned to identifying CVD risk factors, which can be screened for in the history and physical exam. These include age, family history of early CVD, cigarette smoking, impaired glucose tolerance and type 2 DM, hypertension, dyslipidemia, OSA, and obesity [6]. Age seems to be associated with CVD risk. MI risk is increased in the over-45 cohort, high in the over-65, and worse with smoking and hypertension [59]. Postmenopausal PCOS patients have more CAD by angiography [44]. The AE-PCOS Society conducted a systematic review of the literature to identify PCOS–CVD risk relationships [35]. They identified PCOS women with metabolic syndrome, type 2 DM, and/or overt vascular or renal disease to be at highest risk for CVD, and women with any of obesity (especially abdominal), cigarette smoking, dyslipidemia, hypertension, impaired glucose tolerance, a family history of premature CVD (male <55 years, female <65 years), and subclinical vascular disease to be at risk, albeit lower.
NAFLD/NASH
Nonalcoholic fatty liver disease (NAFLD) has a relationship to PCOS and should be evaluated by history and physical exam. NAFLD is characterized by hepatic steatosis not due to alcohol consumption and is the most common liver disorder in the Western world. Nonalcoholic steatohepatitis (NASH) is an extreme form of NAFLD where hepatitis results from steatosis and may progress to cirrhosis and liver failure. NAFLD and NASH share many risk factors with PCOS, such as metabolic syndrome, type 2 DM, and central obesity. The majority of NAFLD patients have PCOS and PCOS patients have high NAFLD association, even with obesity controlled [60,61]. Around 60% of PCOS patients have been found to have NAFLD, and abnormal liver enzymes have been found in 15% of PCOS adolescents [62,63]. Whether or not NAFLD is related to hyperandrogenism is unclear.
NAFLD/NASH may be asymptomatic or present with fatigue, malaise, or dull right upper quadrant pain. Jaundice is rare. A history of NAFLD/NASH should be evaluated through patient history and record. Hepatomegaly may occasionally be detected on abdominal exam. Past serum liver function tests can be evaluated in the patient record or obtained at a later date.
Psychiatric manifestations
PCOS and its clinical manifestations can have a severe psychological impact on patients. Patients with PCOS appear to be at increased risk for depression and anxiety. Therefore, any patient suspected of or confirmed to have PCOS should be questioned about prior psychiatric diagnoses, specifically depression and anxiety. If there is no history, these patients should be screened in office and referred to a psychiatrist if needed [6]. Screening can be done easily with a two-question questionnaire, the PHQ-2, presented in Table 16.4 [64,65]. It is also important to ask about the use of alcohol and drugs, which patients may be using as forms of self-medication.
Over the past 2 weeks, how often have you been bothered by any of the following problems? | Not at all | Several days | More than half the days | Nearly every day |
|---|---|---|---|---|
1. Little interest or pleasure in doing things | 0 | 1 | 2 | 3 |
2. Feeling down, depressed, or hopeless | 0 | 1 | 2 | 3 |
Women with PCOS may be at a four- to eightfold increased risk of depression [66,67]. Patients with depression most commonly complain of fatigue and sleep disturbances, followed by appetite changes and diminished interest in doing things [67]. The predilection towards depression seems to be independent of PCOS features such as obesity, hyperandrogenism, hirsutism, acne, and infertility [6]. However, PCOS features are certainly implicated as well. Body weight primarily decreases quality of life, but hirsutism, ovulatory dysfunction, infertility, sexual behavior, and negative healthcare experiences with the PCOS diagnosis also contribute [68]. A meta-analysis concluded that PCOS patients have a mild tendency towards depression and anxiety which is influenced by BMI [69]. Body image relates directly to depression severity in PCOS patients [70]. Women with PCOS are less likely to identify with a female gender scheme and more likely to view themselves as androgenous [71]. These alterations in gender identification may partially contribute to psychosocial and emotion problems, including depression. Infertility and the worry of being childless may contribute, although a clear link has yet to be established. Further, sex in PCOS patients may be associated with depression. Some research indicates that women with PCOS have reduced sexual satisfaction with regard to orgasm [72].
Anxiety disorders are the most common psychiatric diagnosis amongst patients with endocrine abnormalities, and depression and anxiety often go hand in hand. PCOS patients have a higher prevalence of anxiety symptoms and generalized anxiety disorder (GAD), which likely additionally contributes to the development of social phobias, specific phobias, and panic disorders [73]. It is hard to clearly define the nature of these disorders in this patient population because anxiety disorders often have their onset during adolescence and may develop slowly over time. They frequently follow a chronic, recurring natural history and become clinically recognizable only when they begin to impair functioning, at a relatively advanced stage. Women with PCOS are more likely to have eating disorders, specifically binge eating [67]. This may result from low self-esteem or a reaction to the appetite-stimulating effects of androgens, impairing impulse control.
It is important to have a prompt diagnosis of PCOS in patients. Some research has shown delayed PCOS diagnosis to be associated with depression and anxiety [74]. Moreover, PCOS patients must be continually monitored over time. A longitudinal study showed a high conversion risk to depression over a 1- to 2-year period [75]. Suicide attempts were seven times more common in PCOS patients in a case-control study [76]. Further, anxiety is a chronic, undulating disease process that may require frequent follow-up.
Encourage physical activity in all suspected patients. Less physically active patients have higher depression rates [77]. Improved diet and exercise can improve depression [78]. Supervised eating may reduce the opportunity for binge eating in bulimic patients. In general, correction of the underlying condition will help improve psychological function [9]. Patients should be reminded that PCOS is a true medical issue with symptoms that can be managed. Support should be provided in a non-judgmental manner and patients should be encouraged to practice self-care and seek help when needed.
Infertility
PCOS and infertility are tightly associated. A history of infertility should be carefully documented in any PCOS evaluation. Likewise, PCOS should always be considered in an infertility presentation, as infertility is often the presenting complaint of PCOS. Infertility is a reliable PCOS feature, and may affect more than half of women with PCOS. PCOS women are far more likely to be using reproductive technology, 13% as compared with 1% [79]. Infertility in PCOS patients is suspected to be a consequence of ovulatory dysfunction and irregularity. Oligomenorrhea decreases the number of conception opportunities and cycle irregularity complicates timing intercourse with ovulation. But, as discussed, anovulation is not necessarily complete or static throughout a woman’s life. It is important to remember that PCOS women may conceive a child. Over time, research has shown that a PCOS woman is just as likely to conceive a child as the normal population [80]. Family history is also important to infertility in PCOS. Sisters of PCOS women have more substantial infertility history and have fewer children overall [15]. Obesity should also be noted, as it tends to worsen infertility and decreases effectiveness of fertility treatments [81]. When evaluating women with PCOS for infertility, it is important to keep in mind causes beyond anovulation, such as male factor infertility and tubal occlusion [6].
Pregnancy complications
PCOS is associated with a number of adverse pregnancy and neonatal complications, which have been established by meta-analysis [82]. Evaluation of PCOS should include a thorough history to document complications with previous pregnancies including miscarriage, gestational diabetes, pregnancy-induced hypertensive disorders, cesarean sections, and preterm births. The history should also inquire about neonatal complications, including low birth rate and admission to the neonatal intensive care unit (NICU). The method of conception should be confirmed – natural, ovulation induction, or assisted reproductive technology (ART). Women who require ovulation induction or ART may have a history of multiple births. Because gestational diabetes, preterm delivery, and pre-eclampsias are exacerbated by obesity, all women with PCOS who are planning to get pregnant should have measurements of BMI, OGTT, and blood pressure prior to conception [6].
Recurrent miscarriage
A miscarriage refers to a spontaneous abortion during the first 12 weeks of a pregnancy. There are many reasons for miscarriage and it is currently unresolved whether PCOS carries an elevated risk of miscarriage, and if so, whether this risk is due to PCOS directly, obesity, or use of anovulation medications. Large randomized controlled trials do not support an increased risk of miscarriage [83]. A meta-analysis shows minor support for PCOS leading to increased miscarriage [84]. However, only 8–10% percent of women with recurrent miscarriage have PCOS [85]. With the same infertility treatment, PCOS women have higher miscarriage rates [86]. However, obesity may be the main driver of spontaneous abortion, as obese PCOS women have higher miscarriage rates than non-obese [87]. Clomiphene citrate, a first-line ovulation medication in PCOS, was once thought to contribute to a high miscarriage rate, but this has largely been refuted. Miscarriages in PCOS exhibit fewer chromosomal abnormalities, suggesting a role for other mechanisms such as placental bed thrombosis or endometrial defects.[88,89].
Gestational diabetes mellitus
Pregnancy induces a state of insulin resistance, which may manifest as gestational diabetes mellitus (GDM) if there is overt glucose intolerance. GDM is diagnosed after a 75 g OGTT demonstrates a plasma glucose concentration greater than 92 mg/dL while fasting, 180 mg/dL after 1 hour, and 153 mg/dL after 2 hours. As women with PCOS have an elevated baseline level of insulin resistance, they are at a significantly increased risk for developing GDM [82]. This is independent of obesity [84]. The presence of the disease should be investigated in a history of past pregnancies. GDM presents similarly to type 2 DM with excessive thirst, frequent urination, and fatigue. A large cohort study showed GDM to be two times more prevalent in PCOS than in the regular population [79]. Some studies show women with PCOS to be prone to GDM if they have a past infertility history [90]. Finally, it is important to further evaluate all women with PCOS with an infertility history as they show an increased risk of glucose intolerance after pregnancy [91].
Pregnancy-induced hypertensive disorders
Pregnancy-induced hypertension (PIH) is defined as a blood pressure greater than or equal to 140/90 without proteinuria at more than 20 weeks of gestation. Pre-eclampsia is PIH with proteinuria of greater than 0.3 g/24 hours and its onset is usually accompanied by peripheral edema and occurs during labor, but can be before or even up to 48 hours afterward. Women with PCOS have a higher risk of both hypertensive disorders [82]. The risk of PIH is greater than that of pre-eclampsia. Whether PCOS is directly associated with pregnancy-induced hypertension, or it is the features of obesity or gestational DM that are responsible, is not well established. Insulin resistance may exacerbate the frequency of pre-eclampsia [92].
Preterm birth
Preterm birth is the delivery of a fetus before 37 weeks and its history should also be evaluated in a history of PCOS. PCOS mothers are more prone to preterm births, and this association is maintained even independently of assisted reproductive technology [79,82].
Neonatal complications
Babies born to mothers with PCOS have higher rates of complications and admission to the NICU [82]. Babies born at less than 2500 g are considered to be low birth weight regardless of gestational age. The average birth weight at term is 2500–4200 g. Low birth weight could be due to prematurity or small for gestational age (SGA), which is a fetal index below the 10th percentile after adjustment for gestational age. PCOS mothers tend to give birth to babies that have lower birth weight [82]. Also, there is some evidence indicating that individuals born with a low birth weight are more prone to later PCOS [93]. Therefore, the birth weight of the presenting patient and her offspring is worth considering in a PCOS evaluation. Another consideration is the use of ART in PCOS infertility as ART is associated with low birth weight [94]. Neonatal complications that may contribute to more frequent admission to the NICU include being very preterm at birth, having an increased risk of meconium aspiration, and low APGAR scores at 5 minutes [79].
Neoplasms
The association between PCOS and neoplasms has been contentious, as the link between androgens and carcinogenesis has yet to be fully established. A risk for endometrial neoplasia has been suspected owing to unopposed estrogen during anovulation stimulating endometrial hyperplasia and carcinogenesis. Additionally, PCOS and endometrial carcinoma share the risk factors of elevated BMI, type 2 DM, nulliparity, and late menopause. Systematic review and meta-analysis of observation studies has estimated that the odds of developing endometrial carcinoma are nearly twofold higher in all women with PCOS, and this is increased to threefold in premenopausal women only [95]. However, the evidence is not strong enough to recommend routine assessments of endometrial thickness. Regardless, patients should be asked about unexpected bleeding and spotting.
There is a potential role for breast cancer in PCOS, considering shared risk factors of infertility and obesity. A retrospective study showed breast cancer to be the leading cause of death in PCOS patients [96]. However, the majority of research does not support a clear link between breast cancer and PCOS. Similarly, androgenic stimulation of ovarian epithelial cell proliferation in the pathogenesis of PCOS may contribute to ovarian cancers, although this theory has never been definitely proven. There is evidence for an increased risk of ovarian cancer in premenopausal women, but the data do not support an increased risk overall [95]. Regardless part of the assessment should include a thorough abdominal, pelvic, and breast exam searching for enlargement (uterus) or masses to help exclude neoplasms [9]. The ovaries of most women with PCOS are grossly enlarged, thus bilateral ovarian masses palpated on physical exam may be consistent with PCOS. Ultrasonography may be helpful if the patient’s habitus limits the ability to perform an accurate pelvic exam.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


