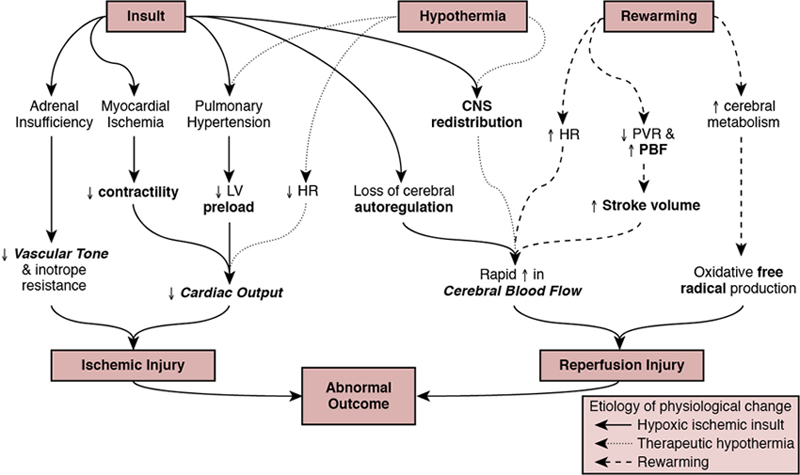
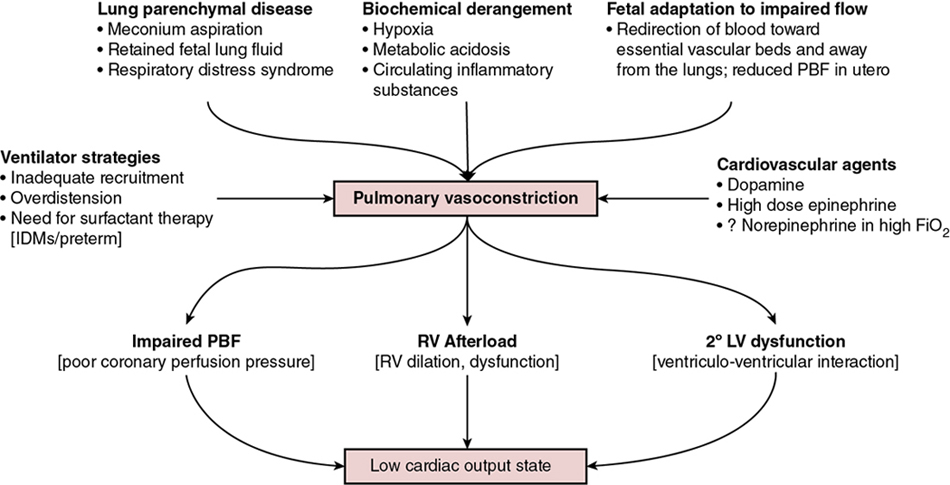
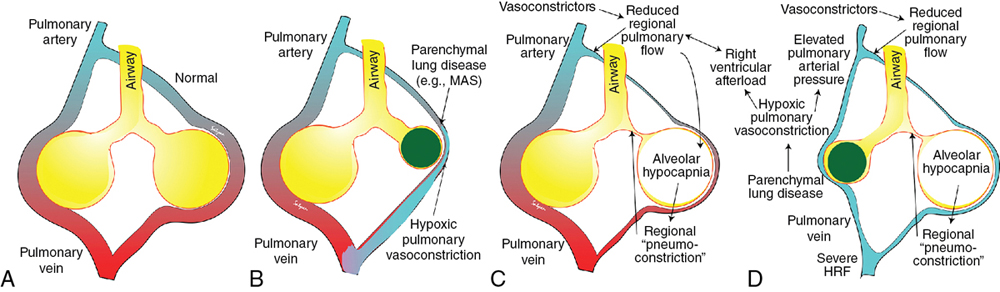
Regan Giesinger, Samir Gupta*, Yogen Singh Globally, neonatal encephalopathy precipitated by perinatal hypoxia-ischemia remains a common cause of brain injury. The incidence varies from 1 to 3 per 1000 to up to 25 per 1000 in developed and developing countries, respectively.1 Therapeutic hypothermia has been demonstrated to improve both survival and neurological morbidity2; however, there remains a significant burden of mortality and long-term neurological sequelae among survivors.3–7 Birth asphyxia, defined as a lack of blood flow or gas exchange to or from the fetus in the period immediately before, during, or after delivery,8 accounts for approximately 1 million deaths per year,6,7 making it a leading cause of infant mortality. In addition, the approximately 1 million children who survive birth asphyxia yearly do so with chronic neuro-developmental morbidities, including cerebral palsy and intellectual and learning disabilities.9 Although the cellular and metabolic contributors to brain injury are complex, abnormal cerebral blood flow is an essential contributor to brain injury by this mechanism (Figure 22.1). In this chapter we will review the interrelationship between the cardiovascular system and the brain, the impact of therapeutic hypothermia on hemodynamics, and management considerations specific to this patient population. The primary determinants of fetal cerebral blood flow are cardiac output, arterial oxygen content, and blood pressure, as has been shown in a fetal lamb model.10,11 Over the range of cerebral autoregulation, the fetal lamb maintains a constant cerebral blood flow despite a wide range of experimentally varied cardiac output (200–700 mL/min/kg).10 Adaptation to low cardiac output involves a complex mechanism that begins with a surge of catecholamines and an associated increase in systemic blood pressure.11,12 This increase in blood pressure is associated with a redistribution of systemic blood flow to the brain.10 Simultaneously, there is an increase in pulmonary vascular resistance which acts to divert more blood from the lungs to essential organs, including the brain, coronary circulation, and adrenal glands. This is facilitated by an increase in right ventricular afterload, which diverts a greater proportion of placental flow from the right to left atrium, and therefore the brain and coronary circulation.13–15 When these and other compensatory mechanisms fail, brain injury may occur. In healthy infants cerebral blood flow (CBF) remains constant over a relatively wide range of changes in systemic mean arterial blood pressure. In animal models the fetal autoregulatory curve differs from that of the adult.16,17 The curve is narrower, particularly at the upper elbow of the curve, and importantly, the normal mean arterial blood pressure in the less mature animal is only marginally above the lower elbow of the autoregulatory curve. In human preterm and term infants the mean blood pressure value at the lower elbow of the autoregulatory curve, or the blood pressure threshold associated with neuronal dysfunction and then tissue injury are unknown.18 Autoregulation is disrupted by hypoxia, hyperoxia, hypocarbia, hypercarbia, and/or acidosis.19 Severe disruption of autoregulation results in a pressure-passive cerebral circulation. In animal models the role of asphyxia-associated impaired CBF autoregulation in the pathogenesis of ischemic cerebral injury has been clearly described. If the same is true for humans, the clinical implications are of importance, as hypotension and decreased cardiac output result in decreases in CBF and thus secondary energy failure and neuronal injury.20 As expected, this process is more pronounced in the more vulnerable regions of the brain, such as the parasagittal cortex or periventricular white matter.17 However, in humans this link between cerebral ischemia and cardiovascular dysfunction is not yet clear, and thus it is not known whether appropriate cardiovascular support and stabilization of CBF improve outcomes in asphyxiated infants. The effects of HI insult on the cardiovascular system are complex, and it is important to understand the principles of developmental cardiovascular physiology and pathophysiology in these sick infants. Both the primary insult and the ongoing redistribution of blood flow can lead to reduced myocardial perfusion, potentially resulting in myocardial ischemia, especially in the sub-endocardial tissue and papillary muscles. Echocardiography studies have consistently demonstrated differences in various measures of wventricular function between patients with HIE and healthy controls, particularly using advanced measurement techniques such as tissue Doppler imaging and speckle tracking.21,22 Structural and metabolic characteristics of the neonatal myocardium confer a greater vulnerability to the effects of hypoxia-ischemia. Specifically, the antioxidant capacity remains underdeveloped at the time of birth, despite upregulation toward late gestation,23 which primes the neonate for oxidative stress during postnatal resuscitation. In addition, ischemia interrupts the normal transition of cardiomyocytes from a glycolytic metabolic pathway utilizing lactic acid to the oxidation of fatty acids.24 This transition is required for efficient energy utilization and adaptation to the oxygen-rich environment. Thus its interruption may confer added vulnerability. Myocardial ischemia may be further complicated by diminished preload, asphyxia-induced autonomic dysfunction, and/or vasoplegia.25 Adequate coronary perfusion, which is often compromised due to hypotension following asphyxia, is critical for optimal cardiac function and may represent an additional metabolic disadvantage. Decreased myocardial contractility may also occur secondary to acidosis and hypoxia.3,26–28 Approximately 25% of neonates with myocardial necrosis on autopsy have a clinical history of asphyxia, making it the most common single cause of perinatal myocardial ischemia.29,30 Transient myocardial ischemia, as diagnosed by electrocardiogram and other non-specific findings, occurs in two-thirds of asphyxiated infants.26 Clinical features may or may not be externally evident26; however, both electrocardiography changes and abnormalities of cardiac enzymes have been linked to the severity of neurological outcomes.3,26,31–33 This supports the importance of monitoring for transient myocardial ischemia, even among so-called “stable” patients. These patients are often normotensive until they are no longer able to compensate and may present with acute cardiovascular collapse. Several factors point to the central role of myocardial ischemia in the pathogenesis of shock among HIE patients. These include higher cardiac troponin T compared to healthy controls32 and the association of elevation in this enzyme with low cardiac output and impaired coronary perfusion.33 That the burden of brain injury is greater among patients with concurrent cardiac dysfunction has also been suggested using echocardiography,34 and as modern evaluative tools become increasingly sophisticated, new associations will become relevant. This includes a recently reported relationship between right ventricular function at 24 hours postnatal age and both short- (i.e., death or abnormal brain magnetic resonance imaging)35 and long-term (survival with neurological impairment)36 outcomes. Although there are important effects of perinatal hypoxia-ischemia in the systemic circulation, the pulmonary vascular impact merits specific consideration. As previously mentioned, part of the fetal adaptive response to impaired CBF includes a redirection of pulmonary blood flow into essential systemic circulatory beds. This, coupled with the fact that neonatal resuscitation efforts are less effective at establishing functional residual capacity than a spontaneously breathing neonate, places the fetus with HIE at risk of failed postnatal transition. Additionally, hypoxia and acidosis are potent pulmonary vasoconstrictors. Finally, neonates with a hypoxic-ischemic injury often have associated morbidities such as meconium aspiration syndrome, lung parenchymal disease, and/or sepsis. These conditions negatively impact lung compliance and/or alter circulating inflammatory mediators, which may independently have a negative impact on cardiac function (Figure 22.2). Pulmonary vasoconstriction has several important downstream effects. First, it produces an afterload stress on the right ventricle, which may exacerbate pre-existing myocardial dysfunction. Second, heterometric adaptation, or dilation, of the right ventricle and septal flattening negatively impact left ventricular function due to ventricular-ventricular interaction. Finally, impaired pulmonary blood flow occurs due to high resistance, compromised right ventricular systolic function, and systemic recirculation of right ventricular output via right-to-left ductal shunt when the ductus is open. This results in compromised left ventricular filling, low left ventricular output, and therefore further impairment of coronary perfusion pressure. Although both ventricles may be affected by perinatal ischemic injury, the right ventricle is particularly vulnerable. There are several putative mechanisms for this. First, the right ventricle plays a dominant role in both fetal and transitional circulation. This results in a higher metabolic demand and therefore a greater requirement for perfusion, oxygen, and other substrate. Second, the conformation of the right ventricle produces a greater circumferential area-to-radius ratio, which results in a greater degree of wall stress for any given afterload compared to the left. Third, when pulmonary artery pressure is high due to elevated pulmonary vascular resistance, the right ventricle may fail to empty normally at the end of systole, leading to increased end-systolic ventricular pressure. When added to diastolic inflow, this gradually increases right ventricular and atrial cavity pressures and reduces the gradient to flow from the aortic root. Aortic root pressure may also be low due to impaired cardiac output. The combination of high right heart pressures and low aortic root pressure leads to impaired coronary blood flow to the right ventricle and impairs the balance of supply and demand of substrate for myocardial metabolism. Perinatal hypoxic-ischemic injury is associated with adrenal insufficiency with or without adrenal hemorrhage. (See Chapter 23 for further details.) In a lamb asphyxia model both ACTH and cortisol are secreted during an asphyxial event as part of fetal compensation. Within 3–5 minutes of interrupted fetal blood flow, fetal adaptation begins to fail and even essential organs have compromised perfusion.37 ACTH levels return to normal with restoration of normal blood flow; however, serum cortisol may remain elevated for hours to days.38,39 Human neonates demonstrate biochemical changes suggesting a shift in adrenal production toward the glucocorticoid pathway.40 Although these studies suggest an intact stress response following asphyxia, a piglet model suggests a delayed response to ACTH stimulation despite high cortisol levels.41 Adrenal hemorrhage and associated adrenal insufficiency have been associated with perinatal hypoxia-ischemia and may be a source of anemia.42 The mechanism is thought to relate to a physical vulnerability to compression/trauma in the setting of a difficult delivery.43 Centralized redistribution of blood to the adrenal glands may confer further vulnerability due to the need to rapidly accommodate a larger blood volume, and the risk of vascular congestion and endothelial damage may also contribute.43 Most adrenal hemorrhages are right sided (70%), with bilateral disease in 10%.43 Bilateral hemorrhages may particularly be associated with adrenal insufficiency. Even in the absence of adrenal insufficiency, postnatal glucocorticoid supplementation may improve dependence on vasoactive medications44 and may play theoretically an important role in capillary leak. In the care of neonates with hypoxic-ischemic encephalopathy, it is essential to remember that therapeutic hypothermia is a post-resuscitation intervention. This is because reduction in core temperature results in predictable cardiovascular changes, which may have deleterious consequences. Both severe and moderate hypothermia have been associated with cardiovascular changes in animal models. In a neonatal lamb model a core temperature of 30°C was associated with a 50% increase in PAP, and even a temperature of 34–35°C has been linked to reduced cardiac output and left ventricular performance in neonatal piglets.45,46 This increase in PVR at lower body temperature may relate to increased vascular tone, circulating catecholamines, and/or the rheological properties of blood, which increases in viscosity at lower temperatures.47,48 Human clinical studies suggest that some neonates experience an increase in requirement for oxygen49 and echocardiography evidence of an increase in PVR50 during TH, which are reversed by rewarming. An increase in clinical pulmonary hypertension was not evident from pooled randomized trial analyses51; however, quantification of pulmonary artery pressure was not systematically performed and neonates with hypoxemic respiratory failure were largely excluded in these large studies.2,52 These factors make it difficult to generalize trial results to the broader population as it relates to cardiovascular presentation. Reduced cardiac output, which occurs in the context of sinus bradycardia due to prolonged ventricular repolarization, has been clearly shown.53,54 It is typical for stroke volume to be relatively preserved.55–57 Among patients with an otherwise healthy cardiovascular system, however, this is matched by lower metabolic rate and typically does not independently result in impaired tissue oxygen perfusion.53 Lower superior vena cava (SVC) flow overall53 with a redistribution of total systemic flow to the central nervous system, as reflected by a greater ratio of SVC:LVO, has been shown to be associated with abnormal MRI.56 This may reflect abnormal cerebral autoregulation; however, clinical relevance needs further exploration. It is reasonable to speculate, however, that relative bradycardia may be either protective or a response to lower cardiac metabolism and may be associated with the lower cardiac troponin I seen following TH in both neonatal animal and human studies.58,59 Given that these changes reverse with rewarming, it may be meritorious to closely monitor systemic and cerebral hemodynamics as temperature is increased, particularly to avoid iatrogenic hypertension. In addition, rewarming affects metabolism and clearance of drugs, including cardiovascular medications.60 The potential cardioprotective effects of therapeutic hypothermia are important to consider, in addition to its neuroprotective effects. In models of adult ischemic heart disease lower metabolism with preservation of energy has been suggested and there is some literature to support positive inotropy61. The isolated ischemic dog heart model has lower ATP consumption at 32–34°C and both rabbit and pig models demonstrate smaller infarct size and lesser post-ischemic LV dysfunction.62–64 TH may also be used in the postoperative management of pediatric congenital heart disease.65 Limitation of reactive oxygen species, inflammation modulation, and the possible mitigation of mitochondrial damage are other proposed mechanisms.66 Given the differences in cardiac metabolism, myocardial composition, and mechanism of injury, neonatal data specific to HIE patients should be considered. Cardiovascular dysfunction among patients with perinatal hypoxic-ischemic injury primarily presents with two phenotypes: with and without hypoxemic respiratory failure. Chapters 25–27 extensively review the evaluation and management of neonatal acute pulmonary hypertension; however, there are several nuances that are specific to the patient with HIE undergoing TH, which will be covered in the following sections. Neonates with HIE have a high intrinsic risk for neurological events, and seizures may present with isolated vital sign abnormalities. Thus brain electrical monitoring using either an amplitude-integrated or full montage electroencephalogram is highly recommended for all neonates, particularly those experiencing abnormal vital signs (e.g., hypoxemia, hypo/hypertension). While heart rate may be useful in the pre-cooling phase, hypoxia-ischemia often leads to compensatory tachycardia or, in more advanced cases, bradycardia related to autonomic dysregulation. Due to the decreased metabolic demand and the direct effects of cooling itself, therapeutic hypothermia is typically associated with a decrease in heart rate in all infants such that a heart rate in the “normal range” may reflect an attempt at compensation for low cardiac output. The use of blood pressure as a metric of cardiovascular health may also be challenging. Therapeutic hypothermia is associated with a reduction in cardiac output.35,67 The low systolic blood pressure, which is associated with reduced cardiac output, may therefore be adaptive. However, among patients with heart dysfunction, low systolic pressure may be an important early sign of cardiac compromise. At the same time, peripheral vasoconstriction may elevate diastolic pressure and mask low mean arterial pressure due to its greater temporal contribution to each beat of the cardiac cycle.53 This is further emphasized by the lack of consensus on a definition of “normal blood pressure”68 in this population and recent evidence that significant heart dysfunction may be present in spite of what would typically be considered normal by most clinicians.69,70 While arterial pressure is an important trending tool, over-reliance may lead to over or under-treatment. As a practical matter, invasive blood pressure measurements are encouraged over noninvasive assessments, due to their suggested higher accuracy in critically ill patients (Chapter 3). If low systolic blood pressure is recorded, it should be correlated with the requirements for oxygen. Normal oxygenation with low systolic blood pressure primarily reflects cardiac systolic dysfunction. On the contrary, the finding of low systolic blood pressure and impaired oxygenation should alert the clinician to evaluate for acute pulmonary hypertension with or without heart dysfunction. Persistent low diastolic arterial pressure should alert the clinician to the potential for vasodilator shock, which may be due to sepsis, adrenal insufficiency, or poorly cleared vasodilating medications (e.g., morphine, milrinone).71,72 To add to the complexity, hypoxic-ischemic injury is often associated with multiorgan dysfunction.4 This makes clinical evaluation of circulatory adequacy using end-organ performance as a surrogate marker challenging for many patients. Both lactate and base deficit have been shown to reflect the severity of the initial insult,73 and rate of lactate decline has limited data showing no association with cardiac output as measured by noninvasive cardiac output monitoring.55 Urine output, similarly, is commonly difficult to interpret. Up to 70% of neonates with hypoxic-ischemic injury are reported to experience acute kidney injury, of which approximately 30% may be classified as severe.4,74 In addition, even among patients with healthy kidneys, therapeutic hypothermia is associated with a temperature-dependent reduction in urine output in a human adult model.75 All things taken together, while clinical assessment is essential and patients should be considered their own controls, the early use of alternative monitoring techniques such as targeted neonatal echocardiography should be considered where feasible. Quantitative echocardiography with objective measures of both left and right ventricular function is recommended early in the disease course. Cardiac dysfunction is common and may be sub-clinical initially, presenting late with an unexpected acute decompensation. This is particularly true among patients with a low burden of associated lung disease. Pulmonary vasoconstriction due to failed transition, therapeutic hypothermia, or a combination of both is common, and in the absence of lung disease the pneumoconstriction occurs in the lungs to ensure that perfused areas are aerated maximally.76 Thus substantial pulmonary vasoconstriction, and therefore high right ventricular afterload, may be present in the absence of clinically apparent pulmonary hypertension (Figure 22.3). Although many centers have integrated echocardiography into the assessment of neonates with HIE either as a screening or diagnostic tool, the majority report using objective measures of LV but not RV performance and CO remains uncommonly reported except in centers with access to TnECHO.68 Targeted neonatal echocardiography may be particularly valuable for integrating ambient physiology into the clinical context and for serial evaluation over time and following intervention. Direct and indirect measures may be used to quantify the relative contribution of right and left ventricular dysfunction, objectify the systemic and pulmonary blood flow, and evaluate the role of shunts. Because of the potential complexity of the physiology in this patient population, it is essential that echocardiography be comprehensive and standardized and performed by trained experts. A detailed review of the evaluation of pulmonary hypertension/right heart performance may be found in Chapter 27; however, our minimum suggested components for a complete evaluation of the patient who has experienced hypoxic-ischemic injury may be found in Table 22.1. Given the prominence of RV disease and the association of abnormal function at 24 hours postnatal age with adverse outcome,35 quantitative assessment of RV performance (Chapter 10) including TAPSE (Figure 22.4) and fractional area change (FAC) (Figure 22.5) should be measured.35
Chapter 22: Hemodynamics of the neonate following perinatal hypoxic-ischemia and the effects of therapeutic hypothermia
Introduction
Fetal cardiovascular adaptation to hypoxia-ischemia
The role of cerebral autoregulation
Cardiovascular effects of hypoxemia-ischemia
Impact on the myocardium
Impact on pulmonary vascular resistance and pulmonary blood flow
Impact of elevated pulmonary artery pressure on cardiac adaptation
Role of adrenal insufficiency
Cardiovascular effects of therapeutic hypothermia/rewarming
Potential cardioprotective role of therapeutic hypothermia
Cardiovascular assessment of neonates with hypoxic-ischemic encephalopathy
Clinical evaluation
Role of echocardiography
Hemodynamics of the neonate following perinatal hypoxic-ischemia and the effects of therapeutic hypothermia



