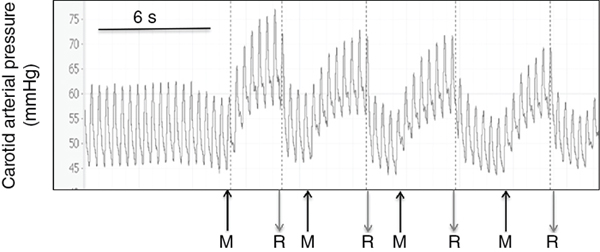
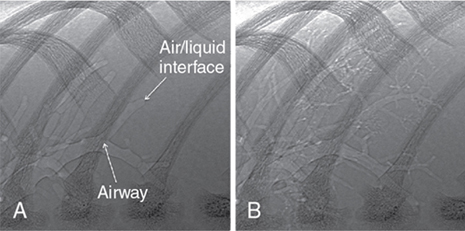
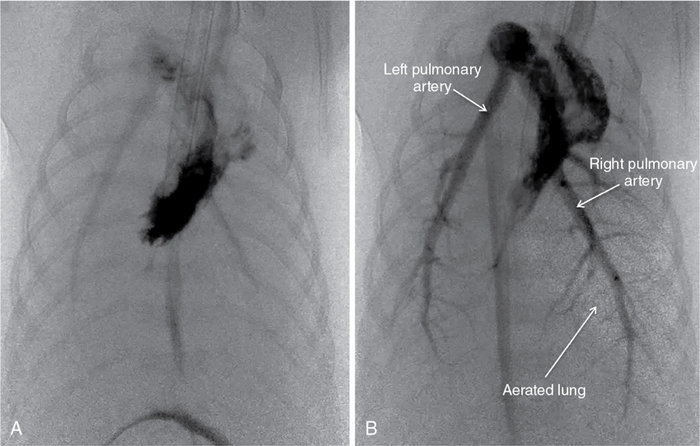
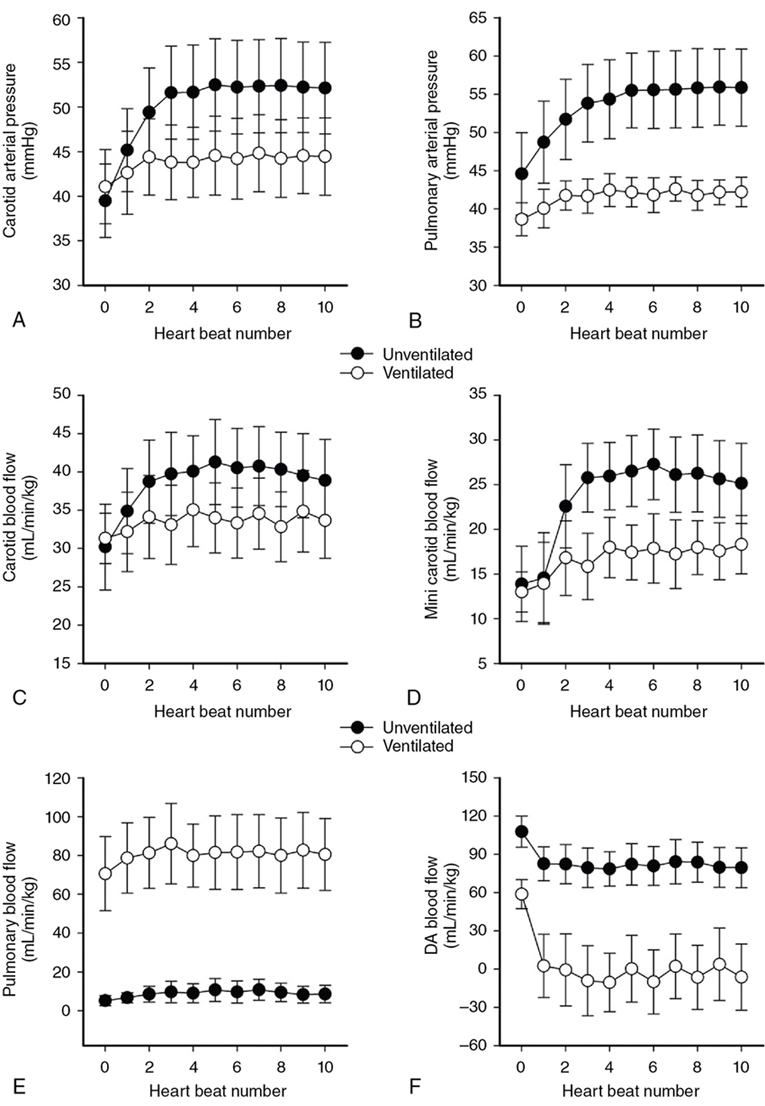
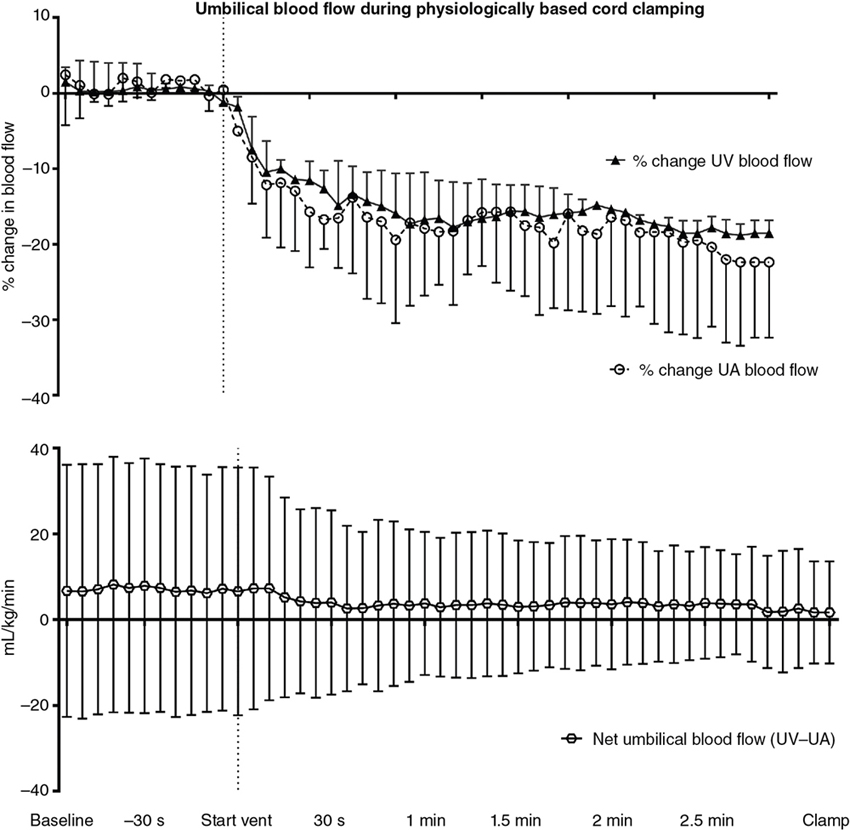
Douglas A. Blank, Stuart B. Hooper, Anup C. Katheria, Martin Kluckow Key points The transition from intra- to extra-uterine life involves a remarkable sequence of physiological events that allow the fetus to survive after birth independent of placental support and in a gaseous environment.1,2 Before birth, the developing lungs are liquid filled and gas exchange occurs across the placenta.3 Cardiopulmonary adaption at birth is triggered by the one event that cannot occur in utero, lung aeration. At birth, the airways must be cleared of liquid to allow the entry of air and the onset of pulmonary ventilation so that gas exchange can transfer from the placenta to the lungs.3 To facilitate the onset of pulmonary gas exchange, lung aeration also triggers a large decrease in pulmonary vascular resistance (PVR). As a result, right ventricular output is redirected through the lungs rather than flowing through the ductus arteriosus (DA), causing a large increase in pulmonary blood flow (PBF).4,5 Before birth, as PBF is low, most venous return and ventricular preload for the left ventricle is supplied by umbilical venous return, which flows via the ductus venosus and foramen ovale directly into the left atrium.6 The increase in PBF plays a vital role in sustaining the infant’s cardiac output by replacing umbilical venous flow as the primary source of left ventricular preload after umbilical venous return is lost due to umbilical cord clamping (UCC). Therefore UCC, before pulmonary ventilation has commenced and PBF has increased, is potentially problematic and causes a large reduction (up to 50%) in cardiac output and increased risk of hypoxemia and ischemia.4,7–9 Thus it is important to understand the physiological changes that occur at birth, as well as be able to recognize the stage within this transitional process that the infant has reached, in order to choose the correct timing for UCC after birth. In utero, the fetus grows and develops in a liquid environment, with gas exchange occurring across the placenta. The future airways are filled with a liquid that is produced by the lung and plays a vital role in stimulating fetal lung growth and development.10 This liquid is actively retained within the airways by the fetus and keeps the lungs under a constant state of distension, resulting in a resting lung volume that is larger than the functional residual capacity of the newborn lung.10 This constant state of distension provides a mechanical stimulus for lung growth, which, if absent, results in severe lung hypoplasia that is either lethal or causes significant morbidity in the newborn.10,11 However, while airway liquid is essential for fetal lung growth, its presence prevents the entry of air and the onset of pulmonary gas exchange after birth. As such, it is important that the airways are cleared as rapidly as possible during the birth process to ensure that air can enter the terminal gas exchange regions of the lung and facilitate the onset of gas exchange. Much interest has focused on the mechanisms of airway liquid clearance at birth, as reduced or variable airway liquid clearance is a major cause of perinatal morbidity, particularly in premature infants or term infants born by cesarean section. There are potentially three mechanisms that can contribute to airway liquid clearance at birth, which depend upon the timing and mode of birth: (i) expulsion of liquid with uterine contractions after rupture of membranes, (ii) increased circulating adrenaline levels during labor which activate amiloride-sensitive Na+ channels and stimulate liquid reabsorption, and (iii) trans-epithelial hydrostatic pressures generated during initial inspirations or mechanical inflations after birth.12 As the fetal respiratory system is highly compliant, small increases in transthoracic pressure, for example, due to postural changes, can greatly reduce the volume of airway liquid. It is well established that the loss of amniotic fluid and uterine contractions increase fetal spinal flexion, which in turn increases both abdominal and intrathoracic pressures, resulting in lung liquid loss.13,14 While this mechanism can account for large reductions in airway liquid at birth, it does not explain how residual volumes of liquid are cleared from the airways after birth. It has been proposed that increased circulating adrenaline levels during labor activate amiloride-sensitive Na+ channels located on the apical surface of pulmonary epithelial cells.15,16 The resulting uptake of Na+ from the lung lumen and its transport across the epithelium into the pulmonary interstitium also increases the electropotential gradient for Cl– ion flux in the same direction.16 This reverses the osmotic gradient driving fetal lung liquid secretion, leading to liquid reabsorption from the airway lumen.16 While this mechanism has been extensively studied and described, it only develops late in gestation and requires very high levels of circulating adrenaline, and at maximally stimulated rates (30 mL/h), it would take hours to clear all airway liquid.12,17,18 As a result, this mechanism likely accounts for only a small proportion (<5%) of airway liquid clearance at birth.19,20 Phase contrast X-ray imaging has allowed researchers to visualize the entry of air onto the lungs at birth in both spontaneously breathing and mechanically ventilated term and preterm rabbits.21–25 These studies clearly show that the air-liquid interface only moves distally during inspiration or during positive pressure inflations (Figure 6.1). Between breaths or inflations, the air-liquid interface either remains stationary or moves proximally, indicating that some airway liquid re-entry may occur between breaths.19,20,25 Based on these results, it was concluded that after birth, airway liquid clearance primarily results from trans-epithelial hydrostatic pressures generated during inspiration/inflation.12,20 That is, inspiration-induced hydrostatic pressure gradients between the airways and surrounding tissue drive the movement of liquid out of the airways across the pulmonary epithelium. This process was found to be extraordinarily rapid, with some newborn rabbits completely aerating their lungs in 3–5 breaths, generating an FRC of 15–20 mL/kg in that time (∼30 seconds).19,20 Similarly, in spontaneously breathing term newborns delivered via cesarean section without labor, 90% had aerated lungs (measured by ultrasound), and 100% had detectable exhaled carbon dioxide within 7 breaths after birth. This confirms a dominant role for inspirations in airway liquid clearance as a single mechanism.26,27 At birth, lung aeration increases PBF 20- to 30-fold,6 which not only enhances pulmonary gas exchange capacity but also plays a critical role in taking over the supply of preload for the left ventricle from umbilical venous return.1,3 Numerous mechanisms are believed to mediate the pulmonary vasodilation in response to lung aeration, including increased oxygenation leading to the release of vasodilators such as nitric oxide, a reduction in lung distension caused by the formation of surface tension, and a vagal-mediated vasodilation caused by the movement of liquid out of the airways into the surrounding tissue.28 The latter mechanism was identified using simultaneous phase contrast X-ray imaging and angiography, designed to examine the spatial relationship between ventilation and perfusion during transition.29 While the imaging was expected to show that partial lung aeration would increase PBF in only aerated lung regions, unexpectedly, the imaging unequivocally demonstrated that partial lung aeration caused a global increase in PBF (Figure 6.2). As ventilation with 100% nitrogen was able to produce a similar response as well as an increase in heart rate,30 it appears that increased oxygenation is not a prerequisite for pulmonary vasodilation at birth, which is a consistently reported finding.31,32 Nevertheless, ventilation with 100% oxygen enhanced the increase in PBF, but only in ventilated lung regions, indicating that the increase in PBF in response to lung aeration is multifactorial, with different mechanisms working independently.30 As vagal nerve section abolished the increase in PBF induced by partial lung ventilation with 100% nitrogen, it was suggested that the movement of airway liquid into lung tissue activated receptors (possibly J receptors), which signaled via the vagus to stimulate a global increase in PBF.33 While the global increase in PBF that is stimulated by partial lung aeration causes a large ventilation/perfusion mismatch after birth, this is not necessarily problematic.29 Indeed, as lung aeration is usually quite heterogeneous,34 restricting the overall increase in PBF by only increasing PBF in aerated lung regions will reduce pulmonary venous return and may affect cardiac output. This is because, following UCC, pulmonary venous return becomes the primary source of preload for the left ventricle and restricting the increase in PBF restricts cardiac output.4 As a result, the importance of clamping the umbilical cord at the appropriate time within this physiological sequence (lung aeration followed by PBF increase) becomes self-evident.3 The fetal circulatory system is very different than that of the newborn and must undergo substantial and rapid changes to transform from a fetal into a newborn phenotype.3,6 Before birth PBF is low, and most of right ventricular output bypasses the lung and enters the descending thoracic aorta via the DA.6 As a result, the left and right fetal ventricles pump in parallel, with both providing output for the systemic circulation. Of note, this circulation also includes perfusing an organ (the placenta) which at times during pregnancy is as big, if not bigger, than the fetus. As the placenta receives a high percentage (30–50%) of fetal cardiac output, umbilical venous return must also provide a large proportion of venous return to the heart, which flows via both the ductus venous and the liver via the IVC.6 Of the umbilical venous return flowing through the ductus venosus, most of this blood bypasses the right atrium, right ventricle, and the lungs by flowing through the foramen ovale into the left atrium.6 This has two important consequences. The first is that highly oxygenated umbilical venous blood can pass directly into the left side of the heart, resulting in higher blood oxygen levels in pre-ductal arteries perfusing the head and upper body.6 The second, often overlooked consequence, is that umbilical venous blood provides a large percentage of the left ventricular preload in the fetus, particularly as PBF is low.1,3 The consequences of umbilical cord clamping at birth are multifactorial. The healthy placenta has a low-resistance, highly compliant vascular bed that receives a large percentage of fetal cardiac output. As a result, clamping the umbilical cord at birth not only separates the infant from its site of gas exchange but also greatly increases systemic vascular resistance and therefore increases afterloads on both the left and right ventricles.4 This causes an instantaneous (within 4 heartbeats) increase in arterial blood pressure (by ∼30%), which results in an equally rapid increase in cerebral blood flow.4 In addition, upon clamping the umbilical cord, umbilical venous return is lost, which reduces left ventricular preload and, combined with the increase in afterload, greatly reduces cardiac output. The loss in cardiac output persists until the lung aerates and PBF increases to restore ventricular preload.35 As such, if this period of reduced cardiac output at birth coincides with even a mild level of birth asphyxia, then the infant is at risk of further hypoxic/ischemic injury. This is because the fetus’s primary defense against periods of hypoxia is to increase and redistribute cardiac output to increase blood flow to vital organs such as the brain.36 However, if cardiac output is reduced, as occurs after cord clamping and before the onset of pulmonary ventilation, then the capacity of the fetus to defend itself from hypoxia is severely limited. Realization that the supply of left ventricular preload must switch from umbilical venous return to pulmonary venous return after birth underpins the rationale for deferring umbilical cord clamping until after the onset of pulmonary ventilation.1,3,4 That is, if PBF increases before the umbilical cord is clamped, pulmonary venous return can immediately replace umbilical venous return as the primary source of preload for the left ventricle without any diminution in supply (Figure 6.3).4 Under these circumstances, umbilical cord clamping does not result in a reduction in cardiac output. It is also important to recognize that the increase in pulmonary venous return following ventilation onset also increases right ventricular output, which increases rapidly with the increase in PBF.4 For this to occur, blood flow through the foramen ovale (FO) must reverse to allow blood to flow from the left into the right atrium and contribute to right ventricular preload. While it has long been considered that the FO is a one-way valve, only flowing from right to left,37 both experimental evidence and clinical observations indicate that this is not entirely accurate.38 Ventilating the lung and reducing PVR before clamping the umbilical cord also greatly mitigates the increase in arterial pressure caused by UCC, because the vasodilated lungs provide an additional low-resistance pathway for blood flow.4,7,9 This initiates a competitive interplay between the pulmonary and placental circulations, whereby flow into either circulation depends upon the downstream resistance (or, more precisely, impedance) in each vascular bed.1,3 As such, following the reduction in PVR, the proportion of right ventricular output passing through the DA into the descending aorta is reduced and redirected into the pulmonary circulation. The result is a substantial reduction in umbilical blood flow entering and leaving the placenta (Figure 6.4).9,39 It is interesting to speculate whether the decrease in PVR contributes to reduced flow and gradual closure of the umbilical vessels after birth. Indeed, anything that alters resistances in either vascular bed will alter the distribution of cardiac output between the two vascular beds and may contribute to umbilical vessel closure when flow into the pulmonary circulation is favored.3 For instance, as uterine contractions increase placental vascular resistance and reduce umbilical blood flow, they increase the distribution of cardiac output into the pulmonary circulation. Similarly, the effect of gravity, caused by positioning the newborn above or below the placenta, induces the same response. That is, following ventilation onset, while umbilical artery flow is reduced as PBF increases, the reduction is much greater if the newborn is placed above the placenta compared with below the placenta.39 UCC following ventilation onset markedly alters the distribution of cardiac output within the newborn.4 The loss of the low resistance placental vascular bed causes a large increase in PBF, due to (1) the redirection of the entire right ventricular output into the lungs and (2) the very rapid reversal of blood flow through the DA, leading to a large left-to-right DA shunt.4,5 As a result, both left and right ventricles contribute to PBF after birth, with the contribution of the left gradually diminishing as the DA closes.5 It has been suggested that this ensures that pulmonary venous return and left ventricular preload is sufficient to maintain or increase (as needed) left ventricular output in the newborn period. It also allows the output of the two ventricles to gradually (over a few hours) come into balance before the two circulations separate, as at that time, the outputs of the two ventricles must be equal.3 Indeed, a continuing but gradually diminishing communication between the two atria (via the FO) and between the two arterial circulations (via the DA) should theoretically facilitate the balancing of the two ventricular outputs. Umbilical cord milking (UCM) is a procedure in which the clinician milks or pushes the blood in the UC from the placenta to the infant. There are two established techniques of UCM that have been described in the literature. One method is “intact umbilical cord milking” (I-UCM). In this technique the clinician milks 20 cm of the UC over 1–2 seconds and releases the umbilical cord after each milk to allow the cord to refill with blood. This process is repeated 2–4 times prior to umbilical cord clamping.40–45 Another UCM technique, called “cut-umbilical cord milking” (cut-UCM), is clamping the UC close to the placenta and milking the residual volume in the UC after cord clamping.46–49 Currently international recommendations discourage the use of UCM outside of clinical studies.50–54 The primary advantage of UCM over deferred UCC is the rapid blood transfer from the placenta to the infant immediately after birth without interfering with evaluation and resuscitation of the newborn. In several small trials authors concluded that UCM conferred the same benefits of deferred UCC.41–43,55–57 In addition, UCM maybe a more effective method to transfer blood during cesarean deliveries because the uterus is not vigorously contracting.43 However, this assumes that the only benefit of deferred UCC is placental transfusion (see below), which it clearly is not, and also neglects the effect that total or intermittent occlusions of the umbilical cord have on the newborn’s circulation. Recent studies have clarified the physiological consequences of UCM procedures.9,58 As expected, UCM necessitates that the cord is occluded during the milking procedure, which causes both a rapid increase in arterial pressure and cerebral blood flow and presumably a decrease in cardiac output, similar to that seen after actual cord clamping.4,9 As arterial pressures are quickly restored following release of the cord to allow it to refill, the resulting changes in arterial blood pressure and cerebral flow in subsequent “milks” are precisely replicated. The net result is a concerning picture of several rapid increases and decreases in arterial pressure and cerebral blood flow, which resemble a “saw tooth” and are in stark contrast to the very stable pressures and flows that occur during deferred UCC. In a newborn infant with either an immature or inflamed cerebral vascular bed, these large “see-sawing” changes in arterial pressures and flows may be of even more concern (Figure 6.5). UCM has been the subject of numerous clinical trials, mostly in near-term infants not requiring resuscitation, and involves milking a 20 cm length of cord over 2–3 seconds, 3–4 times. These trials have suggested that UCM is safe, appears to confer the same benefits of DCC,41,42,55–57,59 and may be more effective in infants delivered by cesarean section.43,60 However, the recent finding of a fourfold increase in severe IVH rates in extremely preterm infants receiving UCM has led to the recommendation against UCM in extremely preterm infants.58,61 There are no published results on the physiologic effects of cut-UCM. The concept that blood volume will automatically shift from the placenta to the infant in a time-dependent manner after birth if UCC is delayed is complex and difficult to explain physiologically. This assumes that throughout deferred UCC, umbilical venous flow will exceed umbilical artery flow, despite the vein being much more susceptible to reduced flow in response to external influences than the artery.1 However, the fundamental principles of circulatory physiology dictate that flow into an organ will always equal flow out of an organ unless there is a major change in vascular compliance (Figure 6.4).9 While a large change in compliance will cause an imbalance in flow, the flow difference will always be transient. As such, blood volume will not automatically shift from the placenta to the infant unless the compliance of the vascular beds within either the infant or the placenta changes.3,35 This is consistent with the fact that fetuses spend ∼9 months in utero perfusing the placenta, and during this time, the distribution of blood volume between the fetus and placenta must be balanced to avoid a continuing net shift of blood from one compartment to the other. Numerous mechanisms have been suggested to enhance placental-to-infant blood transfusion after birth, including gravity, uterine contractions, the increase in PBF, and thoracic pressure changes arising during inspiration.3 On the other hand, an increase in thoracic and abdominal pressures arising from crying or grunting may lead to fetal-to-placental blood transfusion, as would peripheral vasoconstriction possibly caused by birth asphyxia.62 Doppler ultrasound measurements in the umbilical cords of human infants have revealed that breathing, particularly inspiration, and crying have a profound impact on blood flow in both the umbilical arteries and veins.62 Large inspiratory efforts increase umbilical venous flow, leading to an increase in flow through the ductus venosus (and presumably across the foramen ovale), whereas vigorous crying caused flow to cease in both vessels.62,63 In contrast to humans, large inspiratory efforts decrease umbilical venous flow in lambs.64 This discrepancy is most probably anatomical in nature because the ductus venosus enters the IVC before it passes through the diaphragm in sheep, but the ductus venosus does not enter the IVC in humans. As such, it appears that breathing has a major influence on umbilical blood flows that differ between species and more research is required to determine whether this mechanism could drive placental-to-infant blood transfusion in humans. Gravity and Uterine Contractions: While placing newborns below the placenta after birth reduces umbilical artery flow, it does not result in significant placental-to-newborn blood transfusion because umbilical venous flow is reduced by the same amount. On the other hand, placing the newborn above the placenta doesn’t result in infant-to-placental blood transfusion as flows in both umbilical vessels are changed by this procedure.39 These findings are consistent with a clinical trial showing no effect on placental transfusion when placing infants at the vaginal introitus versus on the maternal abdomen.65 Blood volume measurements in human infants suggest that uterine contractions facilitate blood transfusion into the infant after birth.66 However, during labor, uterine contractions are known to increase placental vascular resistance and decrease umbilical blood flow, having differential effects on umbilical arteries and veins.67,68 That is, as the veins are highly compliant low-pressure vessels, during contractions, the veins close earlier than arteries and then reopen after the arteries when the uterus relaxes.67,68 This reasoning explains the bradycardic fetal heart rate response to uterine contractions during labor, causing a transient increase in placental blood volume, which is released back into the infant following the contraction.67 Consequently uterine contractions do not readily explain placental-to-infant blood transfusion, and in particular, they do not “squeeze” blood out of the placenta and into the infant (Figure 6.6).68
Chapter 6: Hemodynamic significance and clinical relevance of deferred cord clamping and umbilical cord milking
The transition to newborn life and lung liquid clearance
Airway liquid clearance
Increase in pulmonary blood flow at birth in response to lung aeration
The cardiovascular transition at birth: Effect of umbilical cord clamping
Neonatal cardiovascular responses to umbilical cord clamping
Neonatal cardiovascular consequences of umbilical cord milking
Placental transfusion during delayed umbilical cord clamping
Potential mechanisms for placental blood transfusion
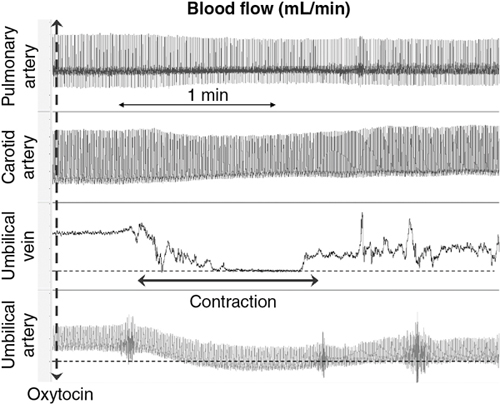
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Obgyn Key
Fastest Obstetric, Gynecology and Pediatric Insight Engine