Elaine Neary, TJ Boly, Regan Giesinger (E) Key points There are several biological conditions that highlight important physiological concepts and require special attention when caring for an infant faced with cardiovascular compromise. As neonatologists, we generally treat a population of patients with structurally normal hearts but abnormal cardiovascular physiology. That is crucial because that makes up the bulk of clinical practice for each licensed independent practitioner every day; however, it is also important to consider how these principles may need to be adapted to other unique situations. In this chapter we will focus on the application of physiological principles to the management of the infant of a diabetic mother with hypertrophic cardiomyopathy, pre-ductal arteriovenous malformation, preterm arterial pulmonary hypertension (PH), congenital diaphragmatic hernia (CDH), and twin-to-twin transfusion. Diabetes mellitus is a common maternal morbidity, associated with higher body mass index, higher maternal age, and sedentary lifestyle, affecting 9–25% of pregnancies.1–4 The term “infant of a diabetic mother (IDM)” refers to neonates born to a woman with persistently elevated blood sugar during pregnancy.5 Infants of diabetic mothers, even with good glycemic control, are at five times increased risk of both morphological and functional cardiac changes because of characteristic fetal metabolic abnormalities such as hyperglycemia and hyperinsulinemia.5–7. Fetal exposure to these conditions often contributes to neonatal morbidity, which may be transient or permanent and predispose to cardiovascular disease in adulthood.1,8 Even asymptomatic infants may have subclinical functional impairment.5 Congenital heart disease, including septal defects, transposition of the great arteries, persistent truncus arteriosus, and patent ductus arteriosus (PDA), is associated with maternal diabetes.1 Cardiomyopathy including hypertrophic obstructive cardiomyopathy, is identified in 13–44% of cases, with the greatest risk associated with maternal type 1 diabetes.9,10 Hypertrophic cardiomyopathy is described as a combination of asymmetric septal hypertrophy and diastolic dysfunction.9,11 These cardiac structural changes may be due to hyperglycemia, activating a cascade of cellular events and changes in gene expression.1 They have also been found to be associated with hyperinsulinemia and IGF-1, which promotes hypertrophy in cardiomyocytes, leading to decreased myocardial compliance.1 Asymmetric septal hypertrophy is typically due to a greater density of insulin receptors in this region as compared to other areas of the cardiac muscle. This may lead to left ventricular outflow tract (LVOT) obstruction and systolic anterior mitral valve displacement.10 Although good maternal glycemic control may improve heart development and mitigate the risk of increased ventricular thickness compared to infants of poorly controlled diabetic mothers,5 it does not eliminate the risk of septal hypertrophy.12 Echocardiography can be utilized to demonstrate structural and functional changes commonly found in IDMs; specifically, it is useful to examine the interventricular septum and posterior wall thickness and measure left ventricular (LV) systolic and diastolic function.13,14 Biomarkers such as cardiac-specific troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-pro BNP) may also be used to predict HOCM and LV dysfunction. cTnI is an inhibitory protein involved in cardiac muscle relaxation released in settings of myocardial injury. Serum levels are correlated with the degree of septal hypertrophy, which may be due to suboptimal coronary artery oxygen delivery to compensate for high myocardial oxygen demand.15 IDM are at increased risk of respiratory distress syndrome because fetal hyperinsulinemia impairs surfactant production.16,17 Lung disease may lead to secondary acute pulmonary hypertension (PH); however, animal studies have also shown increased muscularization of pulmonary arteries and fewer pulmonary vessels at birth, suggesting that a predisposition to pulmonary vascular disease may exist in the absence of lung disease. Maternal diabetes is also associated with chronic fetal hypoxia and polycythemia, which are risk factors for PAH at birth.18,19 Katheria et al. demonstrated that even following well-controlled DM during pregnancy, the IDM is at risk of abnormal transitional hemodynamics. This was supported by lower right ventricular output compared to controls.8 These data are also consistent with the report by Seppänen et al., who showed that the closure of the ductus arteriosus and postnatal decrease in pulmonary artery pressure are delayed in IDMs when compared with control infants during the first postnatal days.6 The consequences of hypertrophic cardiomyopathy may include impaired systemic blood flow and hyperdynamic LV systolic function, which is often observed to coexist with diastolic dysfunction.8 However, even in the absence of hypertrophic cardiomyopathy, functional cardiac changes may be evident. As compared to well-controlled DM pregnancies and healthy controls, IDMs following poor control during pregnancy showed a significant reduction in LV global strain and strain rate. This suggests glycemic control influences the degree of impairment in myocardial systolic function, which persists at least through 6 months postnatal age. Additionally, persistence of the ductus arteriosus may be more prevalent among IDMs following poor glycemic control,5 which may have important implications for preterm IDMs. Interestingly, early functional impairment itself has been suggested to induce cardiac hypertrophy.1 The potential benefit of evaluating fetal cardiac function may lie in its prognostic value for long-term cardiovascular health. Fetal studies have shown that in utero reprogramming of genes involved in metabolic processes can occur secondary to maternal diabetes, especially when associated with a high-fat diet.20 Several biochemical markers associated with adult cardiovascular disease have been identified as higher among IDMs than control infants. These include increased cellular adhesion molecules, markers of endothelial damage, and dysfunctional endothelial colony-forming cells. Elevated levels of angiotensin II, which potentiates stronger vasoconstriction in the presence of endothelin 1, and apoptosis of umbilical venous endothelial cells have been demonstrated in human IDMs.12 While limited longitudinal data specific to the heart is available, these findings may provide biological support to epidemiological studies suggesting a greater burden of cardiovascular disease among IDMs later in life. In most instances infants with hypertrophic cardiomyopathy are asymptomatic and the hypertrophy resolves within months of birth as the stimulus for the insulin production disappears.9,11 A subset of cases, however, develop cardiogenic pulmonary edema and/or shock with severe hypotension due to either LVOT obstruction and/or low LV preload resulting in a low cardiac output. Low cardiac output in these patients is associated with lower cerebral resistance to compensate for low flow through the carotid arteries.21,22 Medication choices involve understanding of the physiology of hypertrophic cardiomyopathy. The most fundamental consideration is that hypertrophic muscle contracts well with often supra-normal systolic function but doesn’t relax well (impaired compliance), resulting in diastolic dysfunction. Practically, this translates into a problem of keeping the LV filled and a need for high LA pressure. During systole, the LV has the capacity to eject and may completely empty before the cavity pressure declines below aortic root pressure. Thus maintenance of high aortic root pressure is one strategy to maintain LV volume. The pattern of deterioration among patients with hypertrophic cardiomyopathy is predictable and should inform the urgency of treatment. The LV becomes underfilled and compensatory tachycardia reduces diastolic duration and further compromises stroke volume. The gradient to flow in the coronary arteries, from the hypotensive aortic root to the hypertensive coronary sinus, is not compatible with coronary blood flow, and the hypertrophic cardiac muscle has a high tissue oxygen demand. The result may be death from cardiac ischemia if prompt management is not instituted to correct the critically low coronary perfusion pressure and either restore LV filling or maintain right to left ductal shunting to support cardiac output. Once a patient becomes significantly hypotensive, emergent intervention should be instituted. The management of acute PH and/ or shock in the infant of a diabetic mother requires modification from routine neonatal intensive care strategies if significant septal hypertrophy is identified, particularly in the presence of LVOT obstruction. The most appropriate approach depends on the degree of obstruction to left heart outflow. If the dominant clinical concern is hypoxia and LVOT obstruction is mild or absent, treatment of PH with strategies that reduce pulmonary vascular resistance (PVR) such as inhaled nitric oxide (iNO) is the most appropriate. Conditions in which the left heart is preload compromised result in closer approximation of the septum and the mitral valve and therefore may exacerbate obstruction. This includes excessive mean airway pressure, particularly in the setting of high-frequency ventilation, where diastolic filling time may be limited. In addition, vasodilator drugs, such as milrinone, should be avoided. Positive inotropes (epinephrine, dobutamine, and dopamine) are contraindicated as more forceful contraction may worsen obstruction and, by inducing tachycardia, may reduce diastolic time, hence further limiting filling. For neonates with hypotension and moderate or severe LVOT obstruction, in whom adequate augmentation of LV filling is not possible, a strategy in which the PVR is kept elevated, and the right heart (via right-to-left ductal shunt) is temporarily used to supply systemic circulation may be the only way to accomplish adequate systemic blood flow. In this case prostaglandin E1 (PGE-1) to maintain ductal patency and avoidance of pulmonary vasodilators is warranted. Veno-arterial extracorporeal membrane oxygenation may be an alternative for neonates with refractory shock, though there is limited published evidence.24,25 In the short term therapeutic hypothermia, which is associated with reduced HR, may be advantageous for these patients; however, this requires prospective study. The changes in heart rate, upon rewarming, in the setting of hypoxic-ischemic encephalopathy and therapeutic hypothermia, may negatively impact the physiology of these patients. Long-term management may be required, though hypertrophic cardiomyopathy in infants of diabetic mothers is transient and typically resolves over 3–6 months. Beta-blockers may improve left heart filling based on their ability to reduce heart rate and for their negative inotropic properties.9 In the acute phase, however, beta-blockers can precipitate symptomatic PH crisis, which may adversely affect LA filling; therefore caution should be exercised. Calcium channel blockers (e.g., verapamil, diltiazem) may also be used to reduce myocardial stiffness. Drugs that reduce systemic afterload (e.g., nitrates, angiotensin-converting enzyme inhibitors, milrinone) should be avoided in this population as they may worsen LVOT obstruction. Diuretics should be used with caution when hypertrophic cardiomyopathy is severe enough to cause pulmonary edema as dehydration may compromise LV filling. There is evidence of a strong association between maternal diabetes and impaired fetal cardiac function in IDMs. Studies directly demonstrating the relationship between fetal cardiac diastolic dysfunction on ultrasound and future cardiovascular health are lacking. There is a need for further longitudinal studies aimed at demonstrating the plausible association between maternal diabetes and fetal cardiac function in utero, in relation to clinical outcomes and offspring development.1 Cardiovascular management is focused on maintenance of LV filling by liberal use of volume boluses and judicious use of vasopressin as the first-line agent for systemic hypotension. In the situation where filling is refractory to fluid and vasopressin, maintenance of ductal shunt to support pulmonary blood flow (and indirectly load the LA) or systemic blood flow (if maintained right-to-left in the setting of severe LVOT obstruction) may be warranted. Arteriovenous malformations are vascular connections that can lead to alterations in the flow of blood to cerebral, pulmonary, and hepatic locations. A vein of Galen malformation (VGAM) is a congenital arterio-venous fistula in the brain that develops in the early embryonic stage resulting in a high-volume pre-ductal left-to-right shunt. Clinical features vary from the differential effects of altered blood flow to the systemic versus pulmonary vascular beds, which can present at different time-points from the early newborn period to late childhood and are associated with increased morbidity and mortality. Infants with VGAM can be asymptomatic initially; however, as the normal postnatal transition occurs, with changing systemic and PVR, infants may become clinically symptomatic with features of poor tissue oxygen delivery. In utero, severe decompensation is rare because the low resistance of the cerebral VGAM is balanced by the low resistance of the placenta, which ensures perfusion of the peripheral organs. With loss of the placenta at birth, up to 80% of cardiac output is directed to the cerebral circulation due to high blood flow through the low-resistance fistula.26–28 Therefore the volume overload caused by VGAM worsens after birth. Patel et al showed superior vena caval flow in this condition may be 10 times higher than that of normal values in their description of three infants with VGAM.26 Higher cardiac output has been associated with higher mortality in the fetus with this condition and may be a useful prognostic indicator in the postnatal period. The right ventricle (RV) suffers from both volume and pressure overload; however, “high-output cardiac failure” is a biological misnomer for the clinical presentation with end-organ dysfunction of patients with VGAM. A more accurate term may be pseudocoarctation because the high flow to the brain acts functionally like an anatomic juxta-ductal obstruction. The high blood flow through the low-resistance fistula can reduce blood flow to the remainder of the body and may result in progressive hypotension, which may be profound secondary to pseudocoarctation physiology.29 Pseudocoarctation physiology is a result of intracranial steal with low coronary root pressure and retrograde transverse and descending aortic arch flow. This means blood flow to the remainder of the body is reduced and can lead to multi-organ ischemia-mediated failure and death. Moreover, myocardial ischemia and infarction have been previously described in these infants. The ischemic myocardial damage stems from a decrease in right coronary blood flow and contributes to the cardiac decompensation. In utero, the fetal vascular bed of infants with VGAM physiology is exposed to substantially more flow than is typical as soon as the capacity of the ductus to shunt blood away from the lung is overcome. This leads to maldevelopment of the pulmonary vascular bed as characterized by pulmonary vascular muscularization and vascular hyperreactivity, which manifests as resistance-mediated pulmonary arterial hypertension (PAH). Intrinsically, patients with VGAM may also present with flow-mediated PAH, given that a substantial volume of blood may fill vessels accustomed to a much smaller volume of flow every minute. It is important to be able to clinically distinguish between these two phenotypes because the principles of management are dichotomous. The former presents with oxygenation failure and typical echocardiographic findings of PAH, while the latter, however, is characterized by a baby who appears generally stable on low (or no) supplemental oxygen but has exclusively or predominantly right-to-left ductal shunt. The lack of compromise to oxygenation relates to the fact these patients have a normal to high right ventricular output (typically low in resistance-mediated PAH), which may be used to distinguish both conditions echocardiographically. A third, mixed phenotype may occur because high pulmonary blood flow over a sustained period can lead to RV dilation and subsequent RV dysfunction due to increased energy demand. This contributes to adverse effects on LV diastolic performance due to abnormal septal configuration and may contribute to left heart–mediated pulmonary edema. The combination of these three cardiovascular phenotypes may lead to multi-factorial oxygenation failure after birth. The presence of supra-systemic PAH may be an indicator of poor prognosis26; however, the relevance of each of these physiologies to a broad population of patients requires further study using comprehensive echocardiography. The physiological contributors to cardiovascular disease among patients with pre-ductal AVMs are highly complex and dynamic. Therapy should be guided by detailed physiological assessment performed serially and a high index of suspicion for changes in ambient conditions. A further consideration is that these neonates are highly vulnerable to brain injury based on abnormal blood flow, vascular distension and compression, and venous congestion. As such, they are at high risk of seizures which could present with subtle physiological deviations, including oxygen desaturation, hyper- or hypotension, etc. Neurological electrical monitoring and empiric phenobarbital use in the setting of sudden decompensation should be considered. Given the bio-physiologic complexity, infants with VGAM should preferentially be admitted to a specialized center with the capacity for both comprehensive longitudinal echocardiography hemodynamic care and for staged endovascular embolization. The goals of management are to (i) maintain a balance of systemic and pulmonary blood flow while providing adequate but not excessive oxygen supplementation, (ii) ensure adequate RV performance, and (iii) assess brain integrity in terms of candidacy for staged embolization. With careful cardiovascular management, emergency embolization should be a rare, if ever, event. This is particularly important because undergoing embolization, with its dramatic changes in loading conditions and physiology, may create an additional risk of poor patient outcomes. The approach to management is dependent on phenotypic characterization of patients with flow-driven PH and resistance-driven PH as treatments such as iNO, which may help the latter, may be harmful to the former. Patients who present with low (or no) supplemental oxygen requirement to maintain pre-ductal SpO2 >85–88% but who have echocardiographic findings which suggest acute PAH (e.g., RV dilation, septal flattening, significant right-to-left component of ductal shunt), are likely to have flow-mediated PAH. In these patients the goal of care is to avoid excessive reduction in PVR, thereby promoting right-to-left ductal flow to direct sufficient blood flow toward the systemic circulation to avoid pseudocoarctation physiology. In addition, iNO is not indicated and may precipitate lactic acidosis and renal failure.29 On the contrary, the initial management of patients with hypoxemic respiratory failure and VGAM should align with the usual approach to resistance-mediated PH with or without RV dysfunction (see Chapter 27). Judicious use of iNO, CO2 targets, and oxygen with optimization of ventilation and sedation are essential components of cardiorespiratory care. In patients without significant lung disease these measures may be very successful in lowering PVR and caution must be exercised to avoid reversal of the (from right to left to left to right) ductal shunt. Weaning to the lowest tolerated dose of oxygen and iNO are important. The aim is to lower pulmonary pressures, to a level that reduces the risk of RV dysfunction by promoting left-to-right flow through the ductus arteriosus, but not at the expense of exacerbating systemic steal and the risk of “pseudocoarctation physiology”. Documentation of normal RV function and treatment of dysfunction with a positive inotrope like dobutamine or low-dose epinephrine is suggested. An intravenous infusion of PGE-1 may also be useful in reducing RV afterload, when RV function is compromised. Hypotension in pseudocoarctation physiology may be due to a closing/restrictive PDA, excessive left-to-right ductal shunt, or RV dysfunction; therefore care should be guided by longitudinal echocardiography. Empiric choices of therapy may include positive inotropy, PGE-1, and shunt modulation strategies such as more liberal CO2 goals, lower target oxygen saturation, and weaning/discontinuation of iNO if applicable. In patients with resistance-mediated PH, low cardiac output is the most likely pathophysiology, and empiric treatment could be either directed toward RV dysfunction or systemic vasoconstriction with agents such as vasopressin or norepinephrine. Caution is advised using norepinephrine concurrently with 100% oxygen and iNO due to its vasoconstrictive effects demonstrated in large animal models.30 Due to the dynamic nature of the physiology, longitudinal targeted neonatal echocardiography (TnECHO) is recommended to guide therapy. The post-embolization period, when multiple feeding vessels are ablated, may be followed by dramatic increases in LV afterload that may lead to impaired LV function. Early post-interventional echocardiography and judicious use of intravenous milrinone may be of value. Infants with VGAM need to be carefully evaluated for signs of PH and systemic hypoperfusion and managed accordingly. Therapeutic goals are to balance systemic and pulmonary blood flow, maintain RV performance, and monitor for neurological abnormalities. Although embolization is the definitive therapy for flow-mediated PH, correction of other ambient conditions is often necessary to achieve optimal operative stability. An algorithm has been published with this in mind by Giesinger et al, as illustrated in Figure 29.1.29 Acute PH occurs in 1.9/1000 live births, with mortality ranging from 4% to 33%.31 The fact that preterm infants, particularly extreme preterms <27 weeks’ gestation, also experience acute PH is increasingly recognized. A recent Japanese cohort study suggests an incidence as high as 18.5% of infants born at 22 weeks’ gestation, with a gradual reduction by maturity thereafter.32 As such, understanding the biological contributors to this disease, the maturity of the physiologic mechanisms required during normal postnatal adaptation, and the impact on both the cardiovascular system and neonate more broadly are important (Figure 29.2). Sections on term acute PH (Chapters 25 and 27) and chronic PH (Chapter 26) may be referenced for more detail. The development of the pulmonary vasculature is a process that occurs concurrently with development of the lung parenchyma. The proximal pulmonary arteries and veins arise from proliferation of endothelial cells from existing vessels in the developing heart.34 The most distal pulmonary vessels develop from pluripotent mesenchymal cells from blood islands which differentiate into endothelial cells.35 The proximal and distal vessels are joined together between 5 and 17 weeks of gestation, during the pseudoglandular stage of lung development.36 As the lungs continue to develop, pulmonary vessels grow alongside each acinus.34 After 20 weeks of gestation, pulmonary arteries and arterioles have completed development of wall musculature.34 The developing pulmonary arterial system has approximately twice the thickness of musculature as compared to those in adults, and the smallest arterioles have more pronounced musculature36; however, there is animal evidence to suggest that arterial smooth muscle controlling vascular tone is mature in later gestation. Studies in the fetal lamb have suggested that the pulmonary vascular bed does not receive increased cardiac output in response to maternal hyperoxygenation at 20 weeks, suggesting the immature pulmonary vasculature does not vasodilate in response to oxygen.37 As gestation progresses to 30 weeks, however, the vasculature does become responsive to maternal hyperoxygenation, suggesting maturation of the vasoreactivity of these vessels later in gestation.34,37–39 Importantly, endothelial nitric oxide synthase (eNOS) is present throughout gestation, suggesting that, if stimulated, it would respond to vasodilatory mediators. Despite the inherent elevated vascular resistance of the fetal pulmonary vessels, several in utero factors may affect the normal development of these vessels and result in a delayed transition or PH following delivery (Figure 29.3). Prolonged premature rupture of membranes and oligohydramnios results in abnormal development of the lungs and alveoli, which in turn leads to poor growth of the pulmonary vasculature with thickened smooth muscle layers.40 Abnormal placentation and intrauterine growth restriction lead to decreased alveolarization, pulmonary vessel density, and endogenous nitric oxide production.41 Maternal factors, including obesity; diabetes mellitus; and use of tobacco, nonsteroidal anti-inflammatory drugs, or selective serotonin reuptake inhibitors, can affect the development of the pulmonary vasculature and its reactivity to endogenous pulmonary vasodilators.42–45 Finally, factors around the time of delivery, including fetal distress and cesarean delivery (both emergent and elective), can contribute to delayed transition to a neonatal cardiopulmonary physiology.38,45
Chapter 29: Hemodynamic management in special circumstances
Introduction
Infants of diabetic mothers and hemodynamic management
Background
IDM and morphological changes
IDM, transition, and functional impairment
IDM and adult life
Hemodynamic management of IDM with obstructive hypertrophic cardiomyopathy
General cardiovascular care for patients with HOCM
Chronic therapy for patients with hypertrophic obstructive cardiomyopathy
Summary
Pre-ductal arteriovenous malformation and hemodynamic management
Background
Systemic consequences of VGAM physiology
Pulmonary consequences of VGAM physiology
Hemodynamic management of infants with pre-ductal AVM
Summary
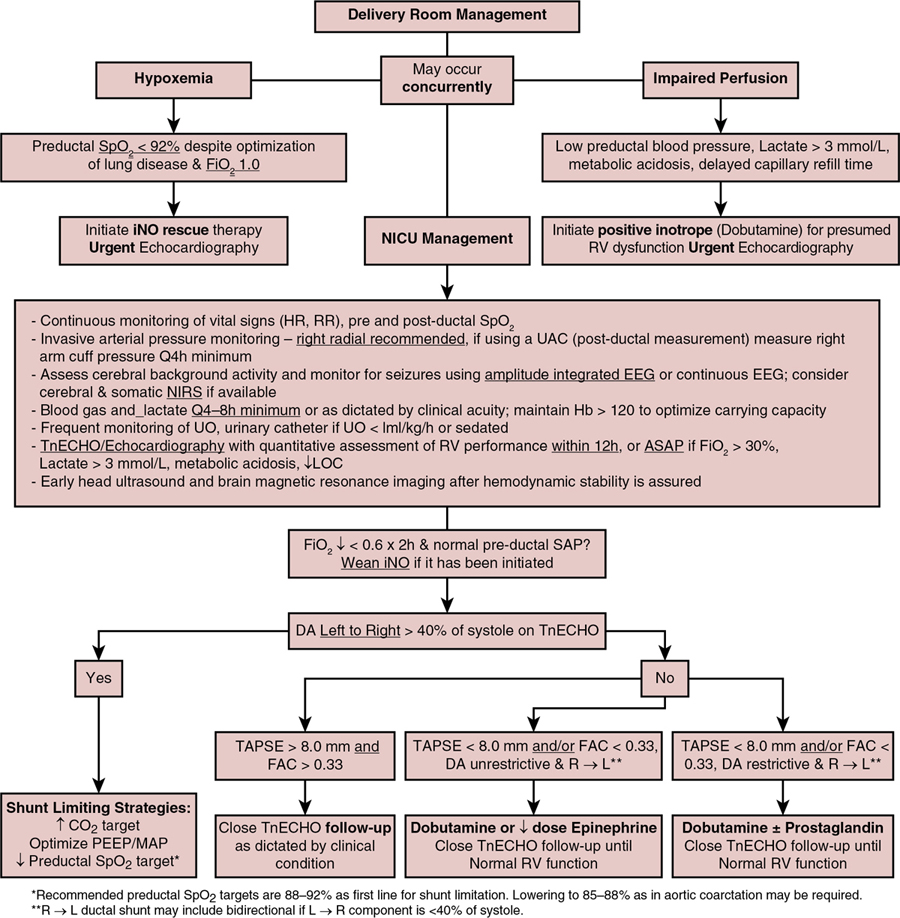
Preterm acute PH and hemodynamic management
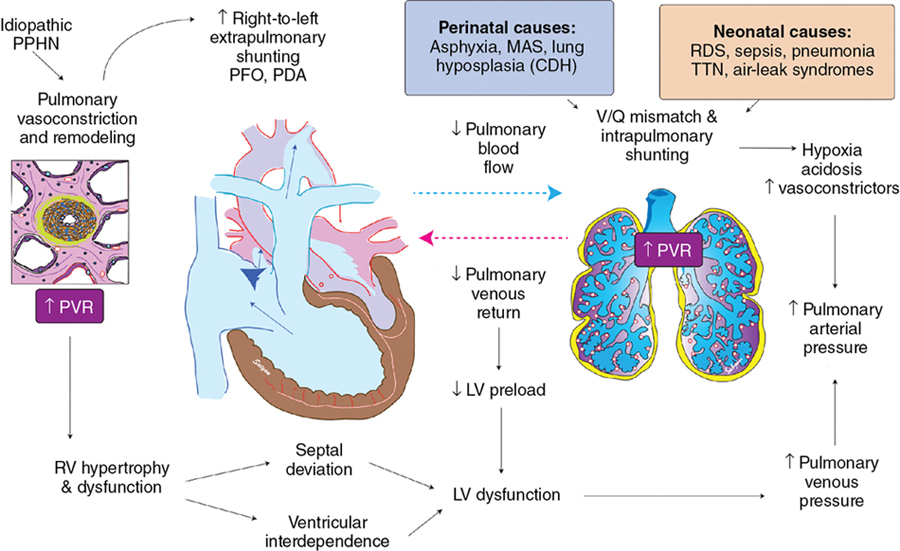
Embryology of the pulmonary vasculature
Pre- and postnatal factors that contribute to PH
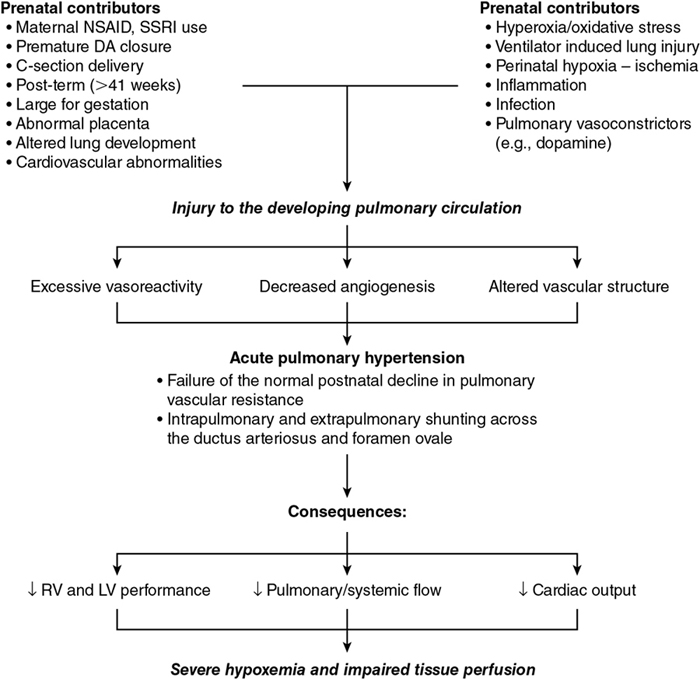
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Obgyn Key
Fastest Obstetric, Gynecology and Pediatric Insight Engine
