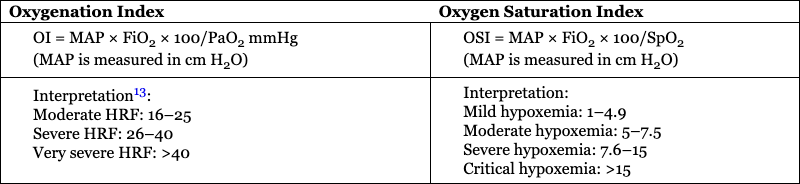
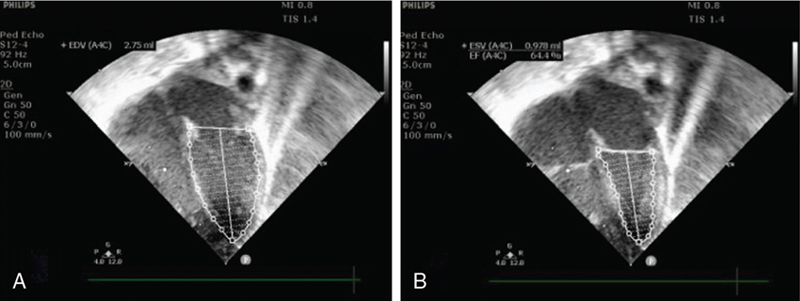
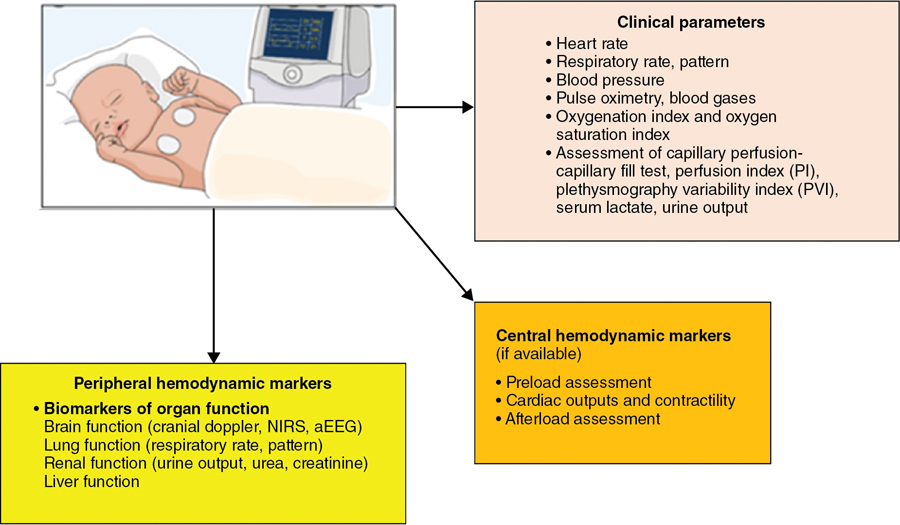
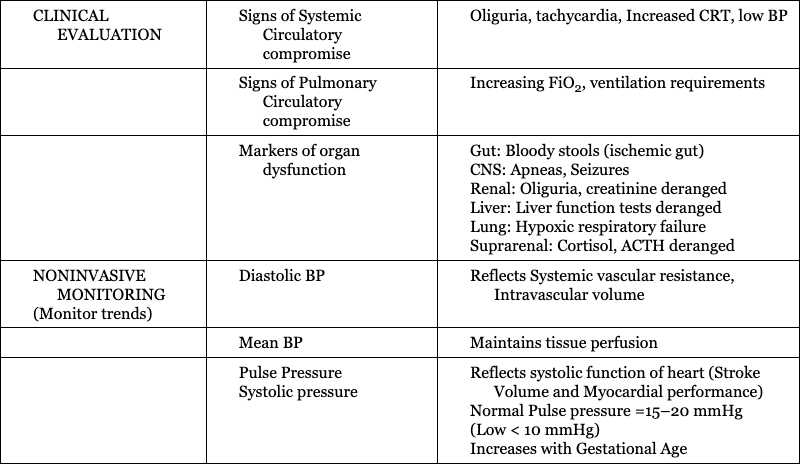
Rema S. Nagpal, Pradeep Suryawanshi, Mohit Sahni Key points Low- and middle-income countries account for 92% of the global disease burden.1 There is tremendous health “inequality” between high-income countries (HIC) and low- and middle-income countries (LMIC) in the provision and availability of health care services, particularly neonatal health care. These inequalities exist due to differences in health financing, availability and training of the health workforce, health infrastructure in the NICUs, and the establishment of safety protocols. An estimated 2.5 million neonatal deaths occur each year, with South Asia and Sub-Saharan Africa contributing to 36% and 43% of these deaths, respectively. HIC countries contribute to <1% of neonatal deaths, highlighting the vast inequalities in the provision of neonatal care.2 Neonatal mortality rates (NMRs) are 25 and 27 per 1,000 live births in South Asia and Sub-Saharan Africa, respectively, while rates are as low as 2–4 per 1,000 live births in many HICs.2 To survive major illnesses, these infants often need highly technical and expensive newborn intensive care, which may not be available in LMIC countries. Hemodynamics encompasses the interaction between heart function, cardiac loading conditions, and the dynamics of systemic and pulmonary blood flow and is controlled by homeostatic mechanisms. The goal of hemodynamic (HD) “monitoring” is to track cardiovascular health over time and aid understanding of the underlying pathophysiology of the disease process, timely detection of hemodynamic compromise, rapid initiation of targeted therapy, and the monitoring of treatment effect. Since the predominant disease processes in LMIC countries include perinatal asphyxia, sepsis (particularly gram-negative sepsis), and prematurity itself, this chapter will concentrate on monitoring the hemodynamic changes in these disease processes. A detailed history is invaluable in providing clues to determine the reasons for clinical deterioration in neonates. Relevant areas include poor antenatal care, signs compatible with intraamniotic infection and preterm premature rupture of membranes leading to early onset of sepsis, delivery before hospital admission/home delivery predisposing to asphyxia and hypothermia, maternal diseases like diabetes which predispose to RDS and congenital heart disease, severe pre-eclampsia/eclampsia predisposing to chronic intrauterine hypoxia and intrauterine growth restriction, and drug intake such as nonsteroidal anti-inflammatory drugs (NSAIDs). A comprehensive clinical examination should be performed including heart rate, respiratory rate and pattern, presence of tachypnea, grunting, blood pressure (BP) measurements and their interpretation, peripheral pulses (to rule out coarctation), pulse volume (as indicators for PDA and warm shock), presence of cyanosis, pulse oximetry, and a pre-post ductal difference in saturations. Indirect clinical assessments like capillary fill time and perfusion index are also helpful. Laboratory assessments using arterial blood gases and lactate levels are useful in the hemodynamic assessment of a sick neonate. However, these clinical parameters are inadequate when used in isolation (Figure 28.1). The objective markers, which are surrogate evidence of the state of cardiovascular stability in neonates, can be divided as follows: “Central” or major markers derived from echocardiography, which assess blood flow and estimated pressure in the central part of the circulatory system (heart, pulmonary artery, vena cava, and aorta), and “Peripheral” parameters, which provide a regional assessment of oxygen delivery, including amplitude-integrated electroencephalogram (aEEG) and near-infrared spectroscopy (NIRS). In resource-limited countries, where echocardiography, aEEG, and NIRS are frequently not available, the clinical parameters have a greater utility and need to be carefully evaluated and interpreted. The commonly used clinical bedside signs of perfusion adequacy in neonates include heart rate, blood pressure, and an assessment of capillary perfusion. However, these markers alone are inadequate in providing accurate information regarding the magnitude of hemodynamic compromise or underlying pathophysiology. Monitoring tools that aid physicians in these countries include: Heart rate is an accurate and routinely monitored parameter in the NICU. Alterations in heart rate (bradycardia <80/min, or tachycardia >180/min) may suggest cardiovascular compromise. Neonates have limitations in their ability to increase the stroke volume; therefore heart rate may increase to compensate for a drop in cardiac output (CO). Since there are multiple other causes for bradycardia/tachycardia in neonates, they are not good indicators of cardiovascular compromise. Routine BP measurement is commonly used as a surrogate for cardiovascular wellbeing, even in HIC countries.3–5 The BP is determined by CO and systemic vascular resistance (SVR). However, an “adequate” mean BP may not equate to an “adequate” CO (see Chapter 3); for example, neonates with high BP due to elevated SVR may actually have a reduced CO. Therefore ideally, CO and BP will need to be monitored simultaneously to determine tissue perfusion. Interpreting BP values, without an objective assessment of cardiac output (using echocardiography), as is followed in many LMICs, may be detrimental in neonates who are critically unwell. In the absence of echocardiography combining parameters like mean BP <30 mmHg and capillary refill time ≥3 seconds increases the sensitivity for detecting measures of low CO such as low superior vena cava (SVC).6 Hypotension, which occurs in one-third of preterm neonates,7 needs vigilant monitoring, due to its effect on the cerebral blood flow auto-regulation, particularly if the mean blood pressure falls to <30 mmHg.8,9 Invasive BP monitoring, which may not always be possible in LMICs, needs adequate staff training, while non-invasive measurements need appropriate cuff sizes10 and tend to overestimate invasive BP values, particularly in immature hypotensive preterm infants.11,12 The oxygenation index (OI) and the OSI are measures that reflect the severity of hypoxic respiratory failure (HRF) in a neonate and are used to trend the degree of oxygen impairment (Table 28.1). The OI is utilized in some centers to determine the need to initiate iNO and is part of the criteria for commencing ECMO.13 Limitations of the OI are that it is invasive, it requires an indwelling arterial catheter, and it is only intermittently performed. OSI is useful in settings where arterial catheter insertion and monitoring are difficult to perform, utilizes the saturation by pulse oximetry (SpO2), and allows for continuous assessment of the severity of the HRF. In studies comparing OI and OSI in ventilated neonates the correlation coefficient compared strongly (0.89–0.95)14,15 and was good in the SpO2 range of 85–95% (r = 0.94). For ranges >95% or <65%, the correlation was poor (r = 0.75).16 OSI correlated more strongly with HRF in preterm infants <34 weeks (<28 weeks, r = 0.93; 28–33 weeks, r = 0.93) compared with late preterm (r = 0.86) and term (r = 0.70) infants.16 OSI can be used to predict various levels of OI,14 making it a potentially useful, noninvasive tool. The regression equation from the derived data showed strong linear association of OSI with OI. A simple relationship between the two measures is that OI values can be derived or predicted from OSI values based on the equation OI = 2 × OSI.14 The strong correlation between OI and OSI makes OSI a useful tool to predict OI in babies with HRF in an LMIC setting. There are, however, some caveats; specifically, SpO2 and PaO2 only correlate linearly when SpO2 ranges between 80% and 97%, but not in the extremes, where OSI may be unreliable. CRT is a commonly used, but unreliable, clinical parameter, which is affected by ambient temperature variations, pressure application, maturity of the neonatal skin, and drugs. A CRT >3 seconds in isolation has a 55% sensitivity and 81% specificity for the prediction of low systemic blood flow, and therefore it is not a good indicator of cardiovascular compromise.6,18 PI is a continuous noninvasive parameter, measured by a pulse oximeter, and calculates the ratio of the pulsatile infrared signal (arterial blood flow) to the non-pulsatile infrared signal (static flow of skin) in the peripheral tissue. It correlates with peripheral perfusion, cardiac output, and stroke volume.19 It reflects the amplitude of the pulse oximeter waveform and is expressed as a percentage (0.02–20%). It is an objective measure of neonatal illness, and the lowest PI is documented at postnatal 12–18 hours.20 Values <1.24 have been identified as an indicator of severe illness in neonates.21 Regional cerebral saturation of oxygenation (rScO2) and cerebral fractional tissue oxygen extraction (cFTOE) also correlate with PI at 24–72 hours.20 PI could be a useful tool in LMIC countries as a measure of perfusion and is already available from the pulse oximeter. (Figure 28.2) A useful, noninvasive parameter, also measured by pulse oximetry, is plethysmography variability index (PVI), which is a dynamic index (from 0 to 100) measuring the relative variability of the plethysmography waveform. Higher variability of the plethysmography waveform indicates preload dependence and the need for fluid administration. PVI is calculated as follows: ((PImax – PImin)/PImax) × 100. It may be affected by clinical deterioration, hypotension, volume insufficiency, or other parameters such as ventilation.22,23 In an Indian study24 that evaluated the changes in PVI in preterm neonates with sepsis and associated hypovolemia, the mean PVI was 28% ± 5 and decreased by 11% ± 5 on resolution of shock. In neonates with associated hypovolemic shock, the average PVI at the onset of shock (31% ± 3) decreased in these infants on the management of hypovolemia by 14% ± 2. PVI correlated positively with the collapsibility of the inferior vena cava (IVC), which suggested need for fluids, though the correlation was weak. Multiple studies25–27 indicate that PVI may be used as a marker of hypovolemia in hemodynamically unstable neonates, particularly in neonates with no access to cardiac ultrasound. Serum lactate is a good indicator of tissue perfusion and tissue ischemia; specifically, plasma levels >2.5 mmol/L might indicate low cardiac output and impaired tissue perfusion.28 Levels may rise before clinical deterioration and worsening serial lactate levels suggest severe disease status and likely higher mortality. The common causes of high lactates include hemodynamic causes of hypoxemia, sepsis, necrotizing enterocolitis, multiple organ dysfunction, and drugs like adrenaline (which increases glycogenolysis and glycolysis in the liver).29 Lactate levels should be used along with other clinical indicators of poor tissue perfusion and not in isolation.30 An important aspect of central hemodynamic monitoring is measurement of cardiac output, which is determined by the heart rate (HR) and stroke volume (SV). An assessment of stroke volume is further determined by the assessment of the preload, cardiac contractility (assessed by echocardiography), and afterload (assessed by systemic blood pressures). Preload assessment in neonates, using jugular venous pressure, is not possible due to the presence of a short neck. Cardiac ultrasound is a useful modality for fluid assessment and responsiveness but may have limited availability in LMIC. Some useful parameters in the echocardiographic assessment include (i) IVC diameter (Figure 28.3) and collapsibility index31; (ii) eyeballing of the left ventricle (LV) with “kissing ventricles” suggesting an underfilled LV31; (iii) reduced ejection fraction by the Simpson biplane method which uses LV end-diastolic areas32; and (iv) variation in the velocity time integral (VTI) at the LV outflow tract (LVOT). A value of >15% predicts need for fluids with a sensitivity/specificity of >90%.33,34 Low bedside BP measurement is a good, albeit late, indicator of volume status. As mentioned above, the plethysmography variability index is a new tool that could be used as an indicator of fluid status in resource-challenged settings. Measurement of LV output equals the systemic blood flow, but only in the absence of a ductal shunt. Measurement of right ventricular (RV) output is reflective of the systemic venous return (in the absence of any atrial shunting). Flow measured in the SVC is normally between 30% and 50% of the total systemic blood flow.35 Echocardiographic assessment of CO,36 if available, is the only available modality in resource-restricted settings to measure CO. Although the intra-observer variability of the measurement is high, and inter-observer variability is even higher, it still remains a helpful noninvasive mode of measuring cardiac output.37–40 Cardiac ultrasound may be used for comprehensive assessment of chamber dilatation, ventricular systolic function (fractional shortening, ejection fraction) (Figures 28.4 and 28.5) and diastolic function, the presence and direction of flow through transitional shunts (ductus arteriosus, foramen ovale), pulmonary artery pressures, cardiac outputs, and fluid status. It is helpful for enhanced characterization of the hemodynamics in neonates with a significant PDA, pulmonary hypertension, perinatal asphyxia and sepsis, and guiding escalation of inotropic/vasopressor or lusitropic support. There is, however, need for a close collaboration with a pediatric cardiology team to rule out undiagnosed structural heart disease.41,42 More detailed description of echocardiographic parameters may be found in Chapters 9 and 10. Afterload is the force against which the heart has to contract in order to eject its stroke volume and depends on the BP, systemic vascular resistance (SVR), and vascular compliance. High afterload situations are seen in the transitional phase on postnatal day 1 (particularly in ELBW neonates) due to clamping the umbilical cord, “cold” shock in sepsis (peripheral vasoconstriction), and at a later time point following PDA ligation. Low afterload is seen in “warm” shock in sepsis and with a patent ductus arteriosus. Measured LV output provides insights regarding the afterload status, as infants with “warm shock” due to sepsis may have high cardiac output. LV afterload or SVR is a derived value from the mean arterial pressure (MAP), CO, and central venous pressure according to the following equation: SVR = MAP/CO (mmHg/L/kg/min) (normal = 150–200 mmHg/L/kg/min). The diastolic BP may also be considered an indirect measure of the afterload status. The adequacy of O2 delivery depends on BP, SVR, and cardiac output. Therefore assessment of the microcirculation is more complex and affected by multiple factors. The assessment of adequate end-organ function may be supported by the following biomarkers: brain function (cerebral dopplers using ultrasound), regional cerebral oxygen saturation (rCsO2) using near-infrared spectroscopy, and amplitude-integrated electroencephalogram, lung function (from respiratory rate, pattern of breathing), renal function (urine output, urea and creatinine), and liver function (using liver enzymes, clotting factors).44,45 The available hemodynamic assessment tools available in NICUs of many LMICs are shown in Figure 28.6. Hypoxic respiratory failure (HRF) with/without pulmonary hypertension (PH) is a complex illness, with rapidly changing hemodynamics, requiring skilled diagnostic ability and resource-intensive management. It is a frequent cause of mortality in LMIC countries. The standard approach to clinical diagnosis and monitoring includes historical clues, comprehensive newborn examination, chest radiography and its interpretation, pulse oximetry for oxygen saturation, and a comprehensive echocardiography assessment.46 Management strategies include optimizing oxygenation, ventilation, and hemodynamics and judicious use of pulmonary vasodilators and inotropes/vasopressors. The consensus opinion of the Neonatal Hemodynamics Working Group (2022)46 suggests that clinicians should adopt a low threshold for comprehensive echocardiography assessment. There is a great deal of heterogeneity in management among NICUs, both in HIC and LMIC countries. Use of inhaled nitric oxide (iNO), high-frequency ventilation (HFV), and extracorporeal membrane oxygenation (ECMO) has greatly revolutionized management and improved survival, but these modalities are expensive and require training, which are not easily available in a resource-limited setting. In a survey done in Canada, Australia, and New Zealand (2008)47 severity of the disease was assessed by echocardiography (86%), arterial blood gas measurements (83%), oxygenation index (78%), and FiO2 (74%). In a subsequent survey (2015)48 the use of echocardiography had increased (95%), and most (77%) used iNO as the first-line pulmonary vasodilator; the estimated mortality was 8.3%. In Indian studies,49,50 in whom the diagnosis of acute PH was confirmed by echocardiography (neonatologist performed or cardiologist performed) but iNO was not available, overall mortality was 29.6%.49 The mortality figures are similar to other data from Asia (20.6%),51 Pakistan (26.6%),52 Egypt (25%),53 and Portugal (32%).54 There are diagnostic and management variations between LMIC and HIC countries. A comprehensive approach to management of neonates with HRF ± acute PH in the NICU includes hemodynamic assessment by integration of the clinical parameters like BP, oxygen saturation, blood gases, lactate (Table 28.2), and comprehensive echocardiography to ascertain the underlying pathophysiology (vasoconstrictor, vasodilator physiology, or mixed picture) (Table 28.3). CLINICAL EVALUATION Signs of Systemic Circulatory compromise Oliguria, tachycardia, Increased CRT, low BP Signs of Pulmonary Circulatory compromise Increasing FiO2, ventilation requirements Markers of organ dysfunction Gut: Bloody stools (ischemic gut) Renal: Oliguria, creatinine deranged Liver: Liver function tests deranged Lung: Hypoxic respiratory failure NONINVASIVE MONITORING (Monitor trends) Diastolic BP Reflects Systemic vascular resistance, Intravascular volume Mean BP Maintains tissue perfusion Reflects systolic function of heart (Stroke Volume and Myocardial performance) Normal Pulse pressure =15–20 mmHg OXYGEN INDICES CARDIAC ULTRASOUND(Rule out CHD) Ventricular systolic & diastolic performance Shunt evaluation- direction, velocity Severity of Pulmonary hypertension DETERMINE DISEASE PATHOPHYSIOLOGY Vasoconstrictor physiology OR Vasodilator physiology MAKE A PHYSIOLOGY BASED MEDICAL RECOMMENDATION FOR MANAGEMENT Clinical Assessment Pulmonary hypertension Suprarenal dysfunction Perinatal asphyxia Medication – anesthetic agents, morphine Cold shock in sepsis ECHO Findings Ventricular outputs Low cardiac outputs High cardiac outputs Systolic performance Impaired systolic performance Normal systolic performance Diastolic performance Impaired diastolic performance Impaired or Normal diastolic performance Shunts Shunts ± Shunts ± Pulmonary pressures Normal or high pulmonary pressures Normal or high pulmonary pressures Systemic vascular resistance (SVR) SVR High Low SVR Management Inotrope (with vasodilatation) Vasopressors NSAIDs Inotropes 1st Line: Dobutamine Milrinone 1st line: Norepinephrine Vasopressin Others – Management Avoid dopamine if pulmonary pressures high Echocardiography remains the gold standard for the diagnosis of PH. In a survey among 148 Indian NICUs55 72% of respondents had neonatologist-performed point-of-care ultrasound (NP-POCUS) services (predominantly cardiac US), while the remaining units had either pediatric (64%) or adult cardiology (33%) services.55 In an Indian study of 187 neonates56 suspected PDA (50%), hemodynamic instability (12.4%), suspected PH (6.6%), and hypotension (13.5%) were the commonest reasons for assessment. The investigators reported that following the echocardiographic assessment, treatment was modified in 42.5% of cases; specifically, addition and/or change in the treatment or avoidance of unnecessary intervention were noted. An increasing number of units from LMIC countries are now adopting clinician-performed ultrasound as a useful hemodynamic assessment tool. The components of the initial clinician-performed ultrasound in neonates with HRF and/or PH are as follows:
Chapter 28: Hemodynamic management in resource-challenged countries
Introduction
Hemodynamic monitoring in neonates
Approach to clinical diagnosis in neonates requiring hemodynamic monitoring
Perinatal history
Hemodynamic assessment tools in resource-challenged settings
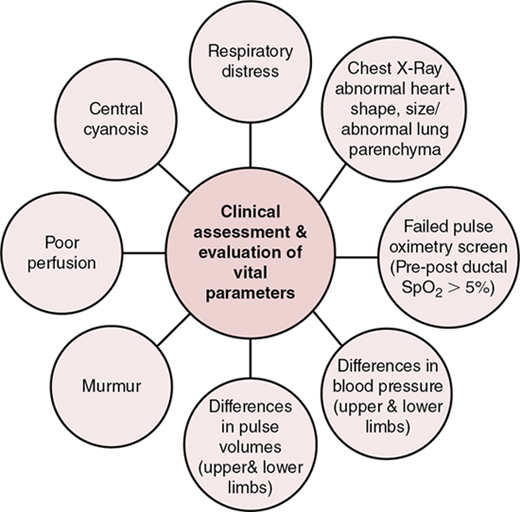
Clinical parameters – utility in hemodynamic assessment
Heart rate
Blood pressure (BP)
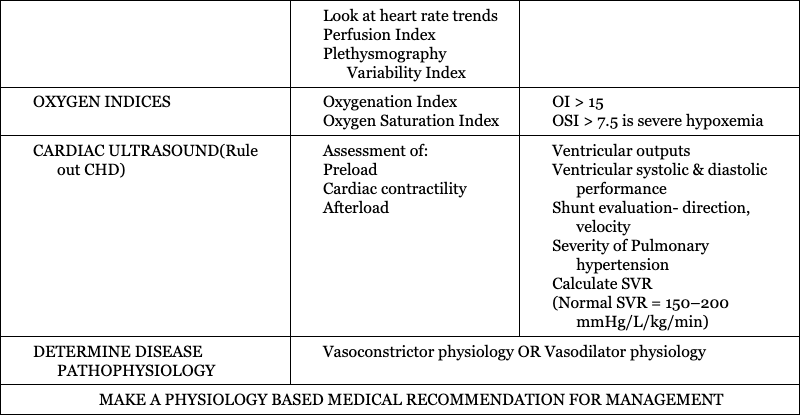
Oxygen saturation index (OSI)
d. Assessment of capillary perfusion17
Capillaryrefill time (CRT)
Perfusion index (PI)
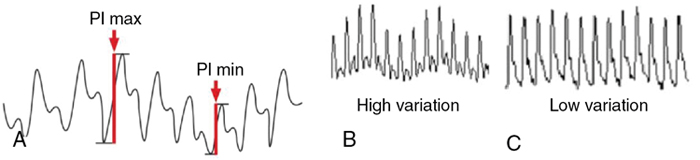
Plethysmography variability index (PVI)
Serum lactate
Central hemodynamic monitoring
Preload assessment
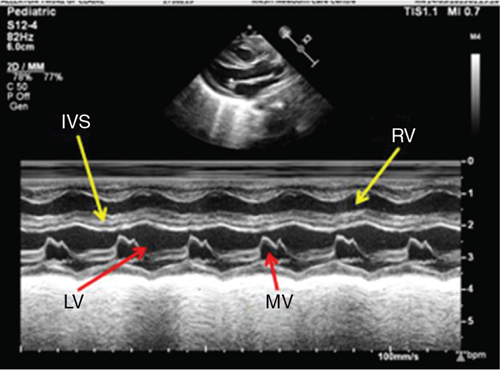
Measurement of cardiac output and contractility
Afterload assessment
Peripheral hemodynamic monitoring and assessment43
Hemodynamic management of a critically ill neonate with hypoxic respiratory failure and acute pulmonary hypertension in LMIC countries
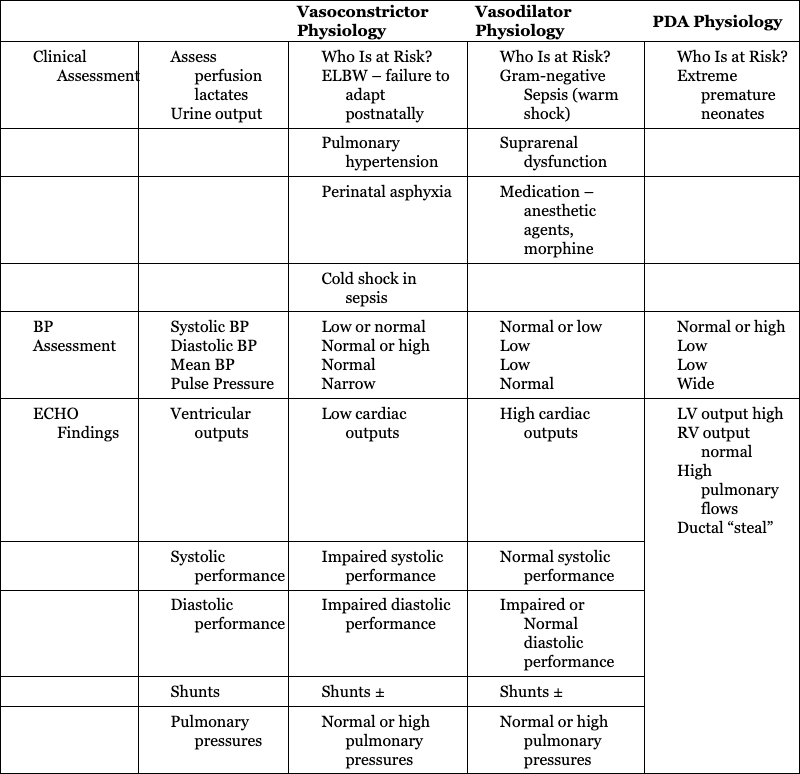
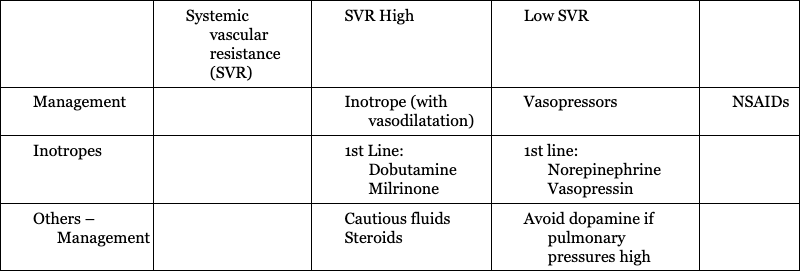
Vasoconstrictor Physiology
Vasodilator Physiology
PDA Physiology
Echocardiographic assessment for neonates with hypoxic respiratory failure and acute pulmonary hypertension
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Obgyn Key
Fastest Obstetric, Gynecology and Pediatric Insight Engine