104 Hematologic Disorders
RBC Disorders
Hemolytic Anemias
Clinical Presentation and Differential Diagnosis
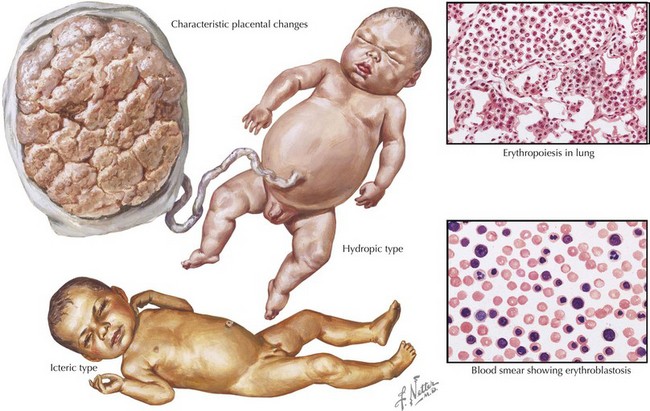
Both ABO incompatibility and Rh disease are associated with jaundice within the first 24 hours of life. In cases of severe hemolytic disease (i.e., erythroblastosis fetalis), infants also present with signs of hydrops fetalis (ascites, pleural or pericardial effusions, edema), pallor (secondary to anemia), petechiae or purpura (caused by thrombocytopenia), and hepatosplenomegaly (result of extramedullary hematopoiesis and splenic sequestration) (Figure 104-1). Each manifestation has a long list of possible causes, but the combination of jaundice and anemia with any of the above findings should focus clinical attention on diseases associated with hemolysis.
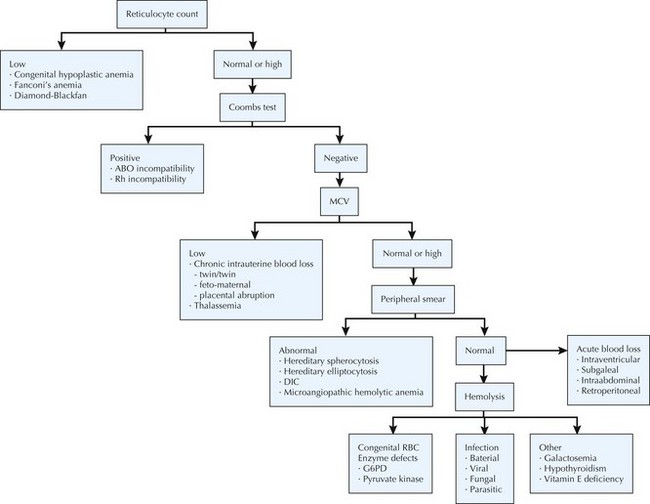
The differential diagnosis of neonatal anemia includes chronic or acute blood loss, congenital disorders of erythrocyte production (e.g., Fanconi’s anemia, Diamond-Blackfan), erythrocyte membrane defects (e.g., hereditary spherocytosis, hereditary elliptocytosis), congenital enzyme deficiencies (e.g., G6PD [glucose-6-phosphate dehydrogenase], pyruvate kinase), infection, and hemoglobin disorders (Figure 104-2). Of the hemoglobinopathies, α-thalassemias are the most common and severe. The switch from fetal (α2γ2) to adult (α2β2) hemoglobin occurs during the first year of life. As a result, defects in α-globin synthesis manifest in utero, whereas defects in β-globin synthesis become apparent in late infancy. Deletion of three (hemoglobin H disease) or four (hemoglobin Barts) α-globin genes can cause significant hemolytic anemia and present as hydrops fetalis. Newborn screening enables early detection and treatment of infants with major hemoglobinopathies and therefore reduces the mortality and morbidity associated with these conditions.
Management and Therapy
Treatment of neonatal hyperbilirubinemia with ET was introduced in the early 1950s. Although ET is proven to reduce mortality and the risk of kernicterus, it is associated with serious complications, including hemodynamic instability, apnea, coagulopathies, electrolyte imbalance, vascular thromboses, sepsis, arrhythmias, and necrotizing enterocolitis (NEC) (see Chapter 100). Efforts to reduce perinatal mortality and the need for ET have led to the development of several prenatal care strategies, including RhoGAM, use of Doppler ultrasound to detect fetal anemia, and intrauterine blood transfusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree