Fig. 24.1
Types of vascular tumors. a Two-month-old female child with a superficial infantile hemangioma (IH) of the cheek. b Five-month-old female child with a deep IH without overlying skin changes. c One-week-old male child with a congenital hemangioma (CH) (rapidly involuting CH) of the neck. d One-month-old female child with PHACE association (Posterior fossa brain malformation, Hemangioma, Arterial cerebrovascular anomalies, Coarctation of the aorta and cardiac defects, Eye/endocrine abnormalities). e Three-month-old female child with a kaposiform hemangioendothelioma. f Five-year-old male child with a pyogenic granuloma
Key Points
Vascular tumors of childhood are typically benign.
Most pediatric vascular tumors can be diagnosed by history and physical examination.
IH is the most common tumor of infancy; most do not require treatment.
Pharmacotherapy is usually the first-line therapy for problematic IH and KHE.
Biology and Epidemiology
Vascular tumors are distinguished by endothelial cell proliferation, whereas vascular malformations result from dysmorphogenesis [1].
Pathophysiology
The precursor cell for IH may be a multipotent hemangioma-derived-stem cell (HemSC) [2]. HemSCs express CD90, a mesenchymal cell marker, and can differentiate into multiple cell lineages. They produce human GLUT1-positive microvessels after clonal expansion in immunodeficient mice [2]. Hemangioma endothelial cells share similarities with placental endothelium, and it has been postulated that the precursor cell for IH might have embolized from the placenta [3, 4].
Several mechanisms may contribute to the rapid enlargement of IH. Hypoxia might recruit circulating hemangioma-derived endothelial progenitor cell (HemEPCs) to the tumor [5]. Hemangioma endothelial cells have defective nuclear factor of activated T-cells (NFAT) activity that stimulates endothelial proliferation [6]. Local factors, such as a reduction in antiangiogenic proteins, also may potentiate tumor growth [7].
Incidence and Prevalence
Age Distribution
The median age of appearance of IH is 2 weeks; subcutaneous lesions may not be noted until 3–4 months of age [10].
Half of KHEs are present at birth, but can develop during infancy (58 %), between 1–10 years of age (32 %), or after 11 years of age (10 %) [14].
The mean age of onset of PG is 6.7 years; only 12.4 % develop during the first year of life [15].
Sex Predilection
Relationships to Other Disease States, Syndromes
A total of 16 % of children with ≥ 5, small, domed-like lesions (hemangiomatosis) have a hepatic IH [18, 19].
PHACE association consists of a plaque-like IH in a segmental/trigeminal distribution with at least one of the following anomalies: Posterior fossa brain malformations, Hemangioma, Arterial cerebrovascular anomalies, Coarctation of the aorta and cardiac defects, and Eye/Endocrine abnormalities (see Fig. 24.1d) [20]. If ventral developmental defects (Sternal clefting and Supraumbilical raphe) are present, the condition is termed PHACES association [20]. Eight percent of patients suffer a stroke during infancy, and 42 % have a structural brain anomaly [21].
Presentation
Infantile Hemangioma
IH grows faster than the rate of the child during the first 9 months of life (proliferating phase); 80 % of its size is achieved by 5 months [22]. After this age the tumor begins to shrink (involuting phase). Regression stops in most children by 4 years of age (involuted phase).
IHs are located on the head/neck (66.5 %), trunk (16.9 %), extremities (9.8 %), or viscera (6.8 %) [9].
Approximately 10 % of IH are problematic during the proliferating phase [10].
Complications:
Alopecia
IHs in the scalp or eyebrow can injure hair follicles.
Obstruction
Ulceration
Approximately 16 % of proliferating IHs will ulcerate at a median age of 4 months; anogenital, labial, and neck tumors are most likely to develop a wound [23].
Deformity
Congenital Hemangioma
Rapidly involuting congenital hemangioma (RICH) regresses immediately after birth; lesions fully involute by 14 months of age [11–13]. Noninvoluting congenital hemangioma (NICH) does not regress [12].
The most common sites of RICHs are the extremities (52 %), followed by the head/neck (42 %), and trunk (6 %) [11, 13]. NICHs affect the head/neck (43 %), limbs (38 %), or trunk (19 %) [12]. After involution, a RICH may leave behind atrophic tissue and/or residual telangiectasia [10].
Most NICHs are nonproblematic, but may cause a deformity [10].
Kaposiform Hemangioendothelioma
Pyogenic Granuloma
The average age of onset is 6.7 years; 72.5 % occur before 10 years of age [15].
Involves the head/neck (80.0 %), trunk (13.3 %), and extremities (6.7 %) [15].
The skin (88.2 %) or mucous membranes (11.8 %) are affected [15].
History of trauma or an underlying cutaneous condition (e.g., capillary malformation) is present in 25 % of patients [15].
The most common complications are bleeding (64.2 %) and ulceration (36.3 %) [15].
Differential Diagnosis for Hemangiomas
Arteriovenous malformation (AVM)
Capillary malformation (CM)
Infantile fibrosarcoma
Infantile myofibromatosis
Kaposiform Hemangioendothelioma (KHE)
Lymphatic malformation (LM)
Pyogenic Granuloma (PG)
Venous malformation (VM)
Diagnosis and Evaluation
Physical Examination
Infantile Hemangioma
Findings:
IH is red when it involves the superficial dermis. Subcutaneous lesions may appear bluish or have normal overlying skin.
Unlike CH, IH is not present at birth and undergoes rapid postnatal growth.
Hand-held Doppler examination showing fast-flow can facilitate the diagnosis [10].
Congenital Hemangioma
Kaposiform Hemangioendothelioma
Laboratory Data
Imaging Evaluation
Ultrasonography (US)
Magnetic Resonance Imaging (MRI)
Pathology
Infantile Hemangioma
Biopsy is indicated only if malignancy is suspected or if the diagnosis remains unclear following imaging studies. Proliferating IH shows disorganized capillary-sized vessels composed of plump, immature endothelial cells [32]. The intervascular stroma is comprised of scattered fibroblasts, mast, and mononuclear cells (see Fig. 24.2) [32]. Tumors in the involuted phase are characterized by fibrofatty tissue, loss of dermal elastic fibers, and small residual capillaries (see Fig. 24.3) [32]. Immunostaining for erythrocyte glucose transporter (GLUT1) can differentiate IH from other tumors and malformations (see Fig. 24.3c) [33].
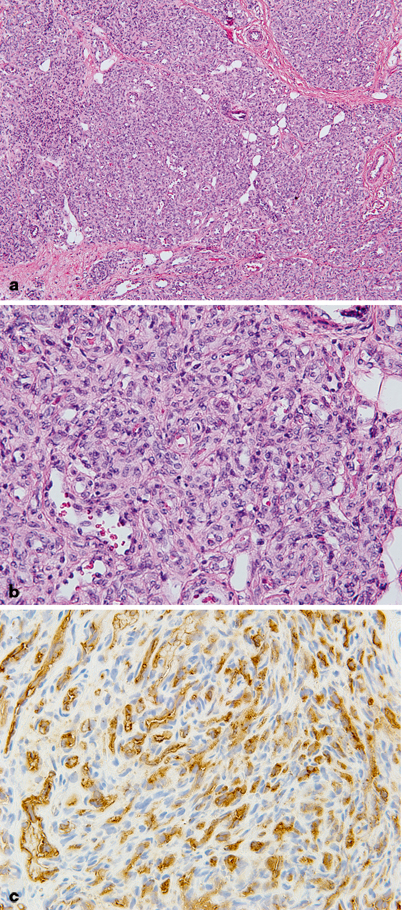
Fig. 24.2
Infantile hemangioma (IH) proliferating phase. a Lobules of capillaries are densely cellular and infiltrating soft tissue. b At higher magnification, capillaries are back to back with little intervening tissue, plump endothelial cells, and difficult to visualized lumens. Mitoses are frequently observed. c Endothelial immunoreactivity for Glut-1 is characteristic of IH
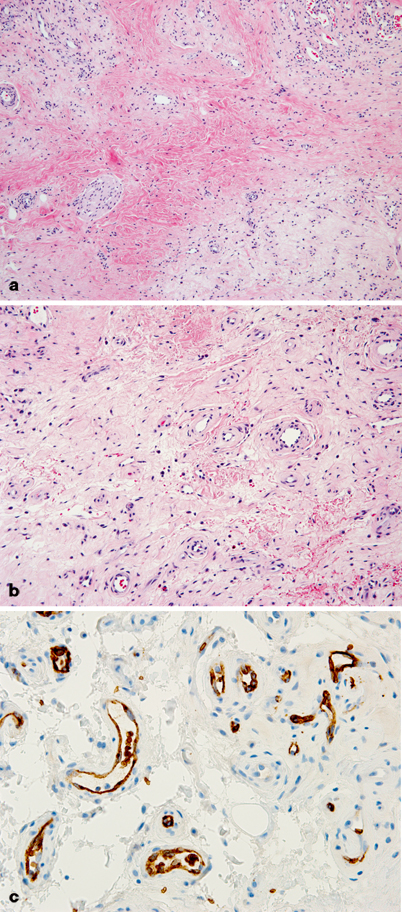
Fig. 24.3
Infantile hemangioma involuting phase. a Capillary lobules are less cellular and vastly replaced by loose fibrous tissue. b Vessels are separated from each other by fibrous tissue and appear larger, and ectatic with thickened walls. c Strong endothelial Glut-1 immunoreactivity persists
Congenital Hemangioma
Biopsy is rarely indicated. Unlike IH, CH is immunonegative for GLUT1. CH shows an accumulation of dermal and subcutaneous lobular capillaries, surrounded by fibrotic tissue (see Fig. 24.4) [32]. Unlike NICHs, the center of most RICHs exhibit involuting features (e.g., fibrotic tissue, residual draining veins, and a lack of capillaries) [32].
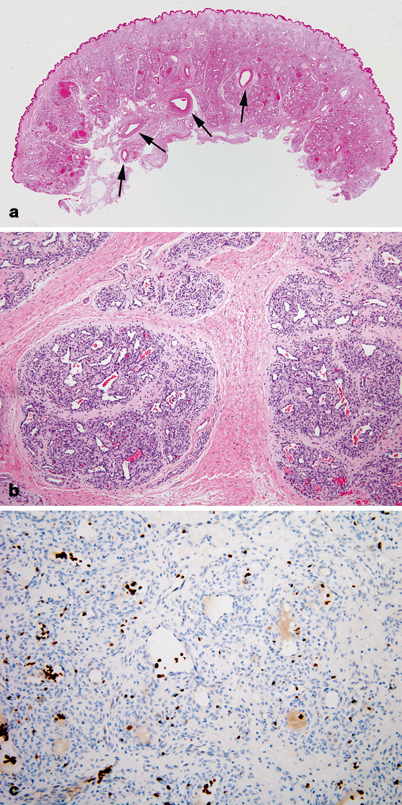
Fig. 24.4
Congenital hemangioma (CH) of the scalp. a CH expanding diffusely into the dermis. Large feeding vessels are present at the base (arrows). b The hemangioma has a distinct lobular pattern of growth. Small vascular channels form the lobules, which are centered by larger channels containing blood. c In contrast to infantile hemangioma, immunohistochemistry for Glut-1 is negative in endothelial cells. Circulating red blood cells are immunoreactive for Glut-1 (dark brown staining cells)
Kaposiform Hemangioendothelioma
Pyogenic Granuloma
On low magnification, PG appears as an exophytic mass attached to a narrow stalk. The superficial lesion demonstrates immature capillaries with fibroblastic, granulation-like tissue [15, 35]. The deeper component contains proliferating lobular capillaries extending to the reticular dermis, with a dense, fibrous stroma [15, 35].
Treatments and Outcomes
Nonoperative Management
Infantile Hemangioma
Most IHs are managed by observation because 90 % are small, localized, and nonproblematic [10]. To reduce the risk of ulceration, proliferating IHs are kept moist with hydrated petroleum to minimize desiccation and accidental shearing of the skin [10]. If ulceration occurs, it will almost always heal with local wound care; if not, intralesional or systemic corticosteroid may be necessary [10].
First-line treatment for small, localized, problematic IH is intralesional corticosteroid (triamcinolone 3 mg/kg) [10]. Topical corticosteroid is less effective than intralesional delivery, because it only treats the superficial component [10, 36, 37]. Corticosteroid injections stabilize the growth of IH in at least 95 % of patients, and decrease the size of 75 % of tumors (see Fig. 24.5a, b) [38]. The drug lasts 4–6 weeks; patients may require additional injections during the proliferative phase.
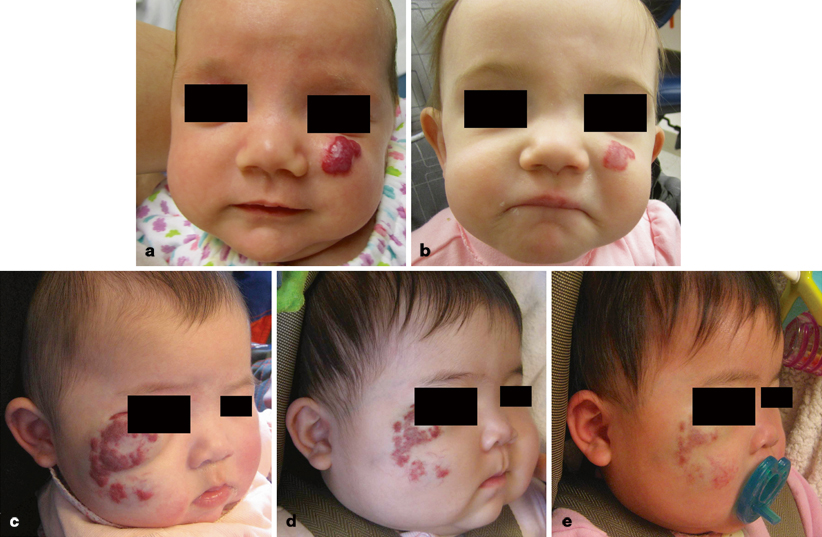
Fig. 24.5
Management of infantile hemangioma (IH) with intralesional or systemic corticosteroid. a Three-month-old female child treated with triamcinolone injection. b Accelerated involution at 12 months of age. c Four-month-old female child prior to initiation of oral corticosteroid. d After 1 month of drug treatment, the IH has regressed. e Age 12 months, after completing corticosteroid therapy
Systemic corticosteroid is reserved for problematic, proliferating tumors that are too large to inject. The child is given 3.0 mg/kg/day of oral prednisolone (Orapred®) for 1 month, followed by a 0.5 ml taper every 2–4 weeks until it is discontinued between 9–10 months of age when the tumor is no longer proliferating [39]. The drug is administered once daily in the morning to facilitate compliance and reduce adrenal suppression [39]. Using this protocol all tumors will respond; 88 % will become smaller and 12 % will stop growing (see Fig. 24.5c–e) [39]. Twenty percent of patients will have a temporary cushingoid response that resolves when the steroid is weaned [39]. Approximately 12–33 % of infants exhibit decreased gain in height, but return to their pretreatment growth curve by 24 months of age [40, 41].
Complications from corticosteroid (e.g., adverse neurodevelopment, aseptic necrosis of the femoral head, diabetes, osteoporosis, adrenal insufficiency, cataracts, and glaucoma) are associated with high-dose, long-term therapy [42, 43] and have not been observed in patients treated with corticosteroid for IH [39–41, 44]. Unlike individuals with hypercortisolism or receiving chronic corticosteroid, children with IH are only treated for several months [39]. In addition, the drug is rapidly weaned as (1) the infant gains weight and (2) the physician lowers the dose [39].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


