4.1 Mild Structural Heart Disease
EXPLANATION OF CONDITIONS
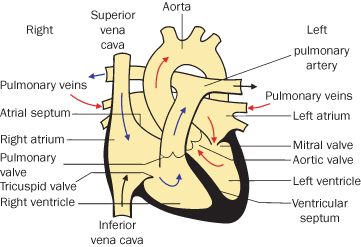
Of children born with congenital heart disease, 85% now survive to adulthood1,3–5. There are a wide range of lesions. Isolated valve lesions are dealt with separately. Other cardiac abnormalities are associated with shunts, missing chambers +/− abnormal connections. Mild conditions are outlined below, moderate and complex conditions in the next sections. Some conditions, especially atrial septal defect, may present or be detected for the first time in pregnancy. Most have previously been repaired but all require consideration and some require further management. Recurrence risks must also be addressed. Congenital heart disease is the most frequent cardiovascular disease presenting in pregnancy with shunt lesions predominating6,7. Figure 4.1.1 shows a normal heart, against which the structural defects can be compared.
Figure 4.1.1 Normal heart
(adapted from Meeks 2010). This figure is downloadable from the book companion website at www.wiley.com/go/robson
Shunts
A shunt is passage of blood through a channel that is not its normal one. Severity is determined not only by the size of the shunt but also the complexity and repairability of associated lesions.
Atrial Septal Defect (ASD)
This is an incomplete closure of the wall between the upper chambers of the heart (left and right atria). It is commoner in women and may be associated with valve problems (see Figure 4.1.2). It is generally repaired in childhood; transcatheter umbrella closure is now common.
Figure 4.1.2 Atrial septal defect, ventricular septal defect and patent ductus arteriosus
(adapted from Meeks 2010). This figure is downloadable from the book companion website at www.wiley.com/go/robson
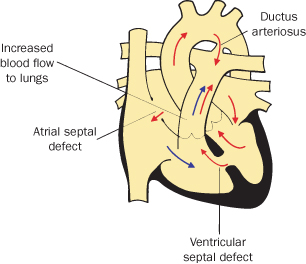
Ventricular Septal Defect (VSD)
Ventricular septal defect is one or more openings in the wall separating the right and left ventricles (see Figure 4.1.2).
It is one of the most common congenital heart defects. About 60% of these close spontaneously or are too small to need surgery; the remaining 40% require open heart surgery, usually in infancy.
Patent Ductus Arteriosus (PDA)
This is the persistence of a normal fetal structure between the left pulmonary artery and the descending aorta. Persistence of this fetal structure beyond 10 days of life is considered abnormal. It is commoner in pre-term infants and is generally closed either surgically or with a transcatheter umbrella-type device (see Figure 4.1.2).
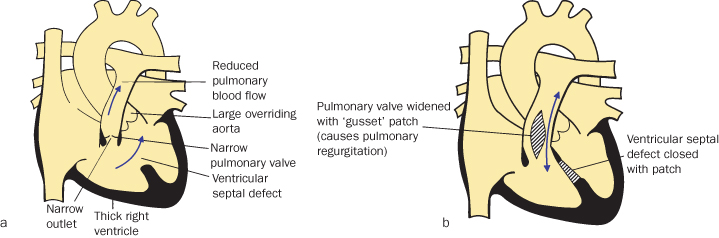
Tetralogy of Fallot
Tetralogy of Fallot has four components:
Tetralogy of Fallot is the most common cyanotic heart defect, representing 5–7% of congenital heart defects. It can be associated with chromosome 22 deletion8, and is generally repaired in infancy or early childhood (see Figure 4.1.3).
Figure 4.1.3 (a) Tetralogy of Fallot
(adapted from Meeks 2010).
(b) Tetralogy of Fallot post trans-annular patch
(adapted from Meeks 2010). This figure is downloadable from the book companion website at www.wiley.com/go/robson
COMPLICATIONS
Atrial Septal Defect
- Atrial fibrillation
- Heart failure
- Stroke
Ventricular Septal Defect
- Congestive heart failure and failure to thrive in infancy and early childhood
- Damage to the heart’s electrical conduction system during surgery (requiring pacemaker or causing late arrhythmias)
- Infective endocarditis
- Aortic insufficiency (leaking of the valve that separates the left ventricle from the aorta)
- Pulmonary hypertension (high blood pressure in the lungs) leading to failure of the right side of the heart9,10 (see Section 4.9 Pulmonary Hypertension)
Patent Ductus Arteriosus
Closure is usually completely successful, but there is an increased recurrence risk for the fetus.
Tetralogy of Fallot
- Post-repair, residual lesions are frequent
- Pulmonary regurgitation is common +/− stenosis, and the valve may need replacement
- Regular cardiological follow-up is essential
- Late arrhythmias also occur
- 22q deletion may not have been recognised previously and has 50% recurrence risk11
NON-PREGNANCY TREATMENT AND CARE
See above
PRE-CONCEPTION ISSUES AND CARE
- Seek expert congenital heart disease specialist advice as to whether further assessment required, e.g. PDA/ASD device closure relatively new
- Assess the requirement for endocarditis prophylaxis (only fully-closed defects are low risk)
- Consideration of pre-pregnancy interventions to optimise maternal and fetal wellbeing in pregnancy for ongoing/residual haemodynamic problems
- Clinical genetics referral for recurrence risk and need for fetal surveillance
- PDA and secundum ASD are normal in utero, hence fetal screening is not prognostic and postnatal echocardiography is recommended
- Other types of ASD (such as partial AVSD), VSD and Tetralogy of Fallot can be detected in utero; mild forms cannot be completely excluded
- Increased recurrence risk in the baby, so specialised cardiac ultrasound screening may be indicated at specialist centres, some as early as 13 weeks12 (see pre-pregnancy counselling)
- Fetal nuchal translucency screening may also be helpful13
- May be newly diagnosed in pregnancy; if repaired, problems are unlikely14
- Open ASD increases risks of arrhythmia, thrombo-embolic stroke and right heart failure
- Previously closed defects very low risk; occasional arrhythmia issues, possible increased risk of pre-eclampsia15
- Small defects near valves have increased endocarditis risks
- Large untreated defects are high risk (see Section 4.9 Eisenmenger’s Syndrome)
- Previously closed/asymptomatic small shunts behave normally
- Large shunts’ ducts can lead to pulmonary hypertension (see Section 4.9 Eisenmenger’s Syndrome)
- 4% recurrence risk postnatally16
- If repaired, pregnancy generally tolerated well but needs cardiological supervision
- For unrepaired defects, see Section 4.3, Severe Structural Heart Disease
- Maternal risk assessment should be carried out according to the modified World Health Organization (WHO) risk classification (see Box 4.1.1)
- Endocarditis prophylaxis no longer routinely required19 but should be discussed on a per patient basis.
- Routine care if previously successfully closed, otherwise consider aspirin thrombo-prophylaxis
- Routine care if previously successfully closed; usual pre-eclampsia screening15
- Small shunts treated as normal, plus endocarditis prophylaxis
- Larger shunts need cardiological supervision
- Co-existing pulmonary hypertension: counselling for ToP etc. (see Section 4.9 Eisenmenger’s Syndrome)
- Arrhythmias may occur
- Closed – treat as normal
- Open or newly diagnosed –follow cardiological advice
- Assess for right outflow obstruction/pulmonary regurgitation, right ventricular function and other residual lesions
- Arrhythmia monitoring and treatment may be required
- Diuretics may be helpful but bed-rest is rarely necessary22
- In the absence of problems a normal vaginal birth is anticipated17
- Early booking with immediate referral to specialist
- Accurate and careful personal and family booking history and baseline observations including respiratory rate and pulse
- Advice on diet and iron supplements to maintain a normal Hb level
- Ascertain need for antibiotic prophylaxis for dental/surgical/obstetric procedures
- Observation for worsening tiredness and breathlessness
- If right ventricular failure develops prepare for a pre-term delivery
- Anaesthetic review prior to labour
- Plan of care drawn up, to include a strategy for oxytoxic drugs18 – generally as normal
- Antibiotic prophylaxis for rupture of membranes or active labour no longer routinely19 required, but should be considered on an individual basis
- Assess individual requirements for antibiotic prophylaxis
- Consider epidural for pain relief
- Define monitoring needs, such as oximetry and ECG
- Define thromboprophylaxis needs, including TED stockings
- Read and follow the care plan thoroughly
- Assist with TED stockings
- Careful monitoring of materno-fetal wellbeing including oximetry as above
- Assess progress in labour, with prompt referral if concerned
- Oxytocin is usually the drug of choice for third-stage management
- ASD – routine care if no complications
- VSD/corrected ToF – monitor fluid balance if potential for congestive cardiac failure (CCF)
- Discuss contraceptive options
- Consider multi-disciplinary follow-up for 6 weeks after delivery23
- Encourage ambulation to reduce risk of thrombo-embolic disease
- Ascertain if maintenance of fluid balance is to be continued
- Reinforce contraceptive advice as above
- Be alert for signs of cardiac disease in the neonate, which might initially present as lethargy or as a feeding problem
- The neonatal examination prior to discharge home is best performed by a paediatrician, not a midwife, due to the risk of cardiac disease
4.2 Moderate Structural Heart Disease
EXPLANATION OF CONDITIONS
Coarctation of the Aorta
- Congenital narrowing of the aorta, generally at the site of ductal insertion, resulting in upper body hypertension and lower body hypoperfusion (see Figure 4.2.1). Aortic wall is often more diffusely abnormal; even with complete and timely repair there is a life-long risk of aneurysm formation and aortic dissection2
- Up to 50% have other heart defects, e.g. VSD, aortic or mitral valve disease (including bicuspid aortic valve)
- Up to 10% of older patients may have cerebral aneurysms at presentation2
- Most coarctations are detected and repaired in infancy or childhood; however, diagnosis in adulthood and even during pregnancy is not uncommon
Figure 4.2.1 Coarctation of the aorta
(adapted from Meeks 2010). This figure is downloadable from the book companion website at www.wiley.com/go/robson
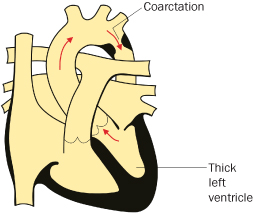
After coarctation repair many children were considered ‘cured’ and were discharged, but there is increasing appreciation of their premature morbidity and mortality3,4 and the need for long-term cardiological supervision.
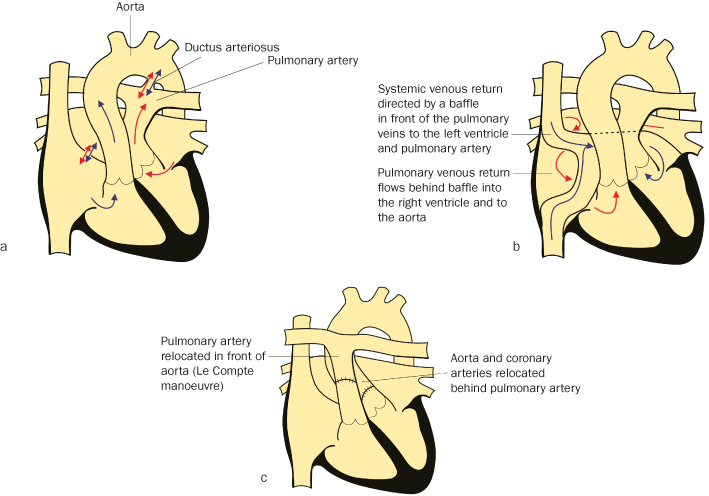
Transposition of the Great Vessels
Like Tetralogy of Fallot, it has been successfully treated for more than 30 years, so there are significant numbers of women with it now entering reproductive life. The right ventricle gives rise to the aorta and the left ventricle the pulmonary artery4 (see Figure 4.2.2a). Initial long-term survivors have generally undergone Senning or Mustard repairs5,6 in which the venous blood is rerouted within the atrial chambers (see Figure 4.2.2b). Current techniques ‘switch’ the great arteries, and coronary arteries to the correct chambers (see Figure 4.2.2c).
Figure 4.2.2 (a) Transposition of the great arteries
(adapted from Meeks 2010).
(b) Transposition of the great arteries post mustard repair
(adapted from Meeks 2010).
(c) Transposition post ‘switch’
(adapted from Meeks 2010). This figure is downloadable from the book companion website at www.wiley.com/go/robson
COMPLICATIONS
Coarctation of the Aorta
- Residual/re-coarctation after repair
- Associated aortic/mitral valve disease
- Hypertension
- Impaired left ventricle function and CCF7
- Thoracic aortic aneurysm/dissection (higher risk in pregnancy)
- Cerebral aneurysm rupture (higher risk in pregnancy)
- Reduced life expectancy7,8
- Infective endocarditis6,9
Transposition of the Great Vessels
Of patients with atrial diversion operations, 30–50% have a degree of systemic ventricular dysfunction and systemic atrio-ventricular valve regurgitation, because the morphologic right ventricle and tricuspid valve are in the systemic circulation10. They are prone to atrial arrhythmias; both tachycardia and sinus node disease can be life threatening and need drugs or pacemaker. Baffle obstruction can occur, and can exacerbate problems from arrhythmias. Following the arterial switch operation there is a potential for coronary artery ostial stenosis and early coronary artery disease. Many patients have branch pulmonary artery stenosis and some have required pulmonary or aortic valve replacement.
NON-PREGNANCY TREATMENT AND CARE
Coarctation of the Aorta
Generally, infants/children are treated surgically, with primary balloon dilatation or stenting used for older children or young adults, as is residual coarctation. Surgery in adulthood entails higher risk, often requiring conduit bypass of the narrowed segment. Hypertension requires aggressive treatment. Aneurysms are increasingly recognised, being managed with surgery or covered stents.
Transposition of the Great Vessels
Post-Mustard (Figure 4.2.2b) or Senning repair, many patients will be on ACE inhibitors +/− diuretics for ventricular dysfunction and systemic A-V valve regurgitation. Stenting for baffle obstruction, the use of atrial pacemakers and antiarrhythmic medication are common. Residual intracardiac shunts, and also pulmonary hypertension, need to be detected and managed.
PRE-CONCEPTION ISSUES AND CARE
Appropriate counselling regarding pregnancy should:
- Start in adolescence
- Give accurate, individual advice correcting misinformation about both contraception and pregnancy
- Identify potential effects of the defect on pregnancy in terms of maternal and fetal risks
- Discuss the effects of cardiac disease including risks of long-term deterioration, and even dying, and whether these will change with time or treatment
Coarctation of the Aorta
- MRI scan prior to pregnancy to exclude aneurysm11 or significant obstruction; deal with if present
- Genetic referral for abnormalities such as 22q deletion
- Recurrence risk is around 4%, and fetal cardiac surveillance is recommended
Transposition of the Great Vessels
- MRI scan and echocardiogram to assess baffles and systemic ventricle or valve function
- Stent baffles if narrowed
- Holter monitoring for rhythm, and drugs/pacemaker initiated if required
- Cease ACE inhibitors
- Control BP with beta-blockers and avoid ACE inhibitors12
- Consider elective caesarean section before term in case of aortic aneurysm formation or uncontrollable hypertension14
- Avoid balloon angioplasty in pregnancy12, but stenting is probably safe
- Monitor for signs of heart failure and arrhythmia15
- Diuretics for oedema
- Beta-blockers for tachycardias but may then need pacing
- Systemic venous baffle obstruction may need stenting
- Early booking with immediate referral to a maternal medicine clinic and re-referral to the cardiologist
- Accurate booking history with baseline observations, especially pulse
- Check blood pressure is correct (usually right) arm as the left sub-clavian artery is often involved in coarctation repair
- Monitor blood pressure regularly for prompt identification of hypertensive disease, especially with coarctation of the aorta
- Maintain Hb levels, so give advice on diet and iron supplementation
- Advise on antibiotic treatment for any dental or other procedures
- Prepare the mother for the possibility of a pre-term delivery
- Antibiotic prophylaxis for rupture of membranes or active labour is no longer routinely required, but should be considered on an individual basis
- Epidural analgesia may be useful as tachycardia secondary to pain increases cardiac work. Care with blood pressure and cardiac rhythm management is important
- Pushing increases vagal tone and may worsen bradycardia
- Assessment for antibiotic prophylaxis
- Antihypertensive drugs may be needed to treat hypertension
- Prophylactic (even temporary) pacemaker may be considered for some transposition of the great arteries (TGA) patients
- If normal birth anticipated consider shortening the second stage
- If there is evidence of aneurysm formation, caesarean section is preferable for delivery13
- Epidural use is recommended
- The midwife might be able to conduct a normal delivery in uncomplicated cases, with an emphasis on a short second stage, which could require an episiotomy
- Alternatively the role might be to assist with an operative delivery, which may well be pre-term
- Otherwise the care is as in Section 4.1
- As with mild structural defects (see Section 4.1) the neonate risks heart disease and requires screening
- The mother with TGA may be at increased risk of venous thrombo-embolism, but for most the risk is normal
- The same contraceptive issues arise as in Section 4.1
- Review medications prior to discharge: some beta-blockers are excreted less in breast milk
- Blood pressure often settles rapidly
- Systemic ventricular dysfunction may continue or worsen post-partum so careful review pre-discharge is required
- ACE inhibitors may be restarted
- Cardiac follow-up 4–6 weeks post-delivery for problematic patients
- This mother is not for discharge until reviewed by the medical team
- Basic post-operative care is required if post-caesarean section
- Basic observations might need to be continued for longer than usual, with an emphasis on blood pressure and fluid balance
- Advise and support specific to the needs of a mother with a baby on a neonatal unit if the delivery was pre-term (see Chapter 1)
- The basic care is the same as in Section 4.1
4.3 Severe Structural Heart Disease
EXPLANATION OF CONDITIONS
These are conditions in which pregnancy carries a significant risk (≥2–5%) for mother and/or fetus, i.e. pregnancy is either high/very high risk or contraindicated. Inevitably, despite counselling, some women will become/wish to remain pregnant. These women require intensive supervision, with which some will achieve an acceptable outcome. This includes women with:
- Unoperated/palliated cyanotic or complex congenital heart disease, including single ventricle conditions
- Women with poor systemic ventricular function (see Section 4.6 Cardiomyopathy)
- Women with Eisenmenger’s syndrome (see Section 4.9)
- Women with Marfan’s syndrome (see Section 4.5)
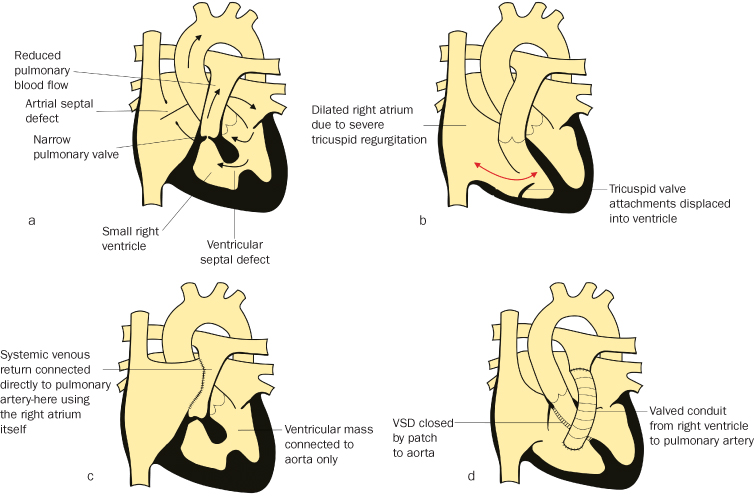
Unoperated Cyanotic Heart Disease
Many women will be recent immigrants. Rarely, cyanotic heart disease may not be diagnosed until adulthood, including in the UK. Some conditions are balanced and may have been managed conservatively deliberately. Lesions include uncorrected Tetralogy of Fallot, pulmonary stenosis/atresia with ventricular septal defect, functionally univentricular heart conditions such as tricuspid atresia (Figure 4.3.1a) and Ebstein’s anomaly with ASD (Figure 4.3.1b).
Figure 4.3.1 (a) Complex – tricuspid atresia
(adapted from Meeks 2010).
(b) Complex – Ebstein’s anomaly
(adapted from Meeks 2010).
(c) Complex – tricuspid atresia post-Fontan operation (adapted from Meeks 2010). (d) Transposition, VSD post Rastelli
(adapted from Meeks 2010). This figure is downloadable from the book companion website at www.wiley.com/go/robson
Palliated Complex Heart Disease
Increasing numbers of children with complex cardiac abnormalities, including both single ventricle conditions and non-septatable VSD, +/− transposition, are surviving to adulthood with a Fontan circulation (see Figure 4.3.1c). This is a ‘final common pathway’ of surgeries for conditions in which the heart pumps blood solely around the systemic circulation, with the systemic venous return passing to the pulmonary arteries without being pumped by a ventricle. It permits separation of the arterial and venous sides of the circulation (hence most are ‘pink’), and also offloads the heart, but pulmonary blood flow is dependent on the venous pump (from the calf muscles), respiratory ‘suction’ and gravity as well as a low-resistance circuit. Many of these patients are anticoagulated to reduce the possibility of thrombosis of rather sluggish pulmonary arterial circulation and increasing numbers are taking diuretics +/− ACE inhibitors. Atrial arrhythmias are common, and some will have pacemakers +/− anti-arrhythmic drugs. Some may have had palliative shunt operations or intermediate/partial Fontan surgery, and hence remain hypoxic to some degree. Some patients will have had repairs involving conduits from the right ventricle to the pulmonary artery (e.g. Rastelli repair for transposition; Figure 4.3.1d) which may become stenosed or regurgitant and require replacement.
COMPLICATIONS
During pregnancy the fall in systemic vascular resistance and rise in cardiac output exacerbates any right-to-left shunting, worsening pre-existing cyanosis and hypoxia. The required increase in cardiac rate and output may also worsen the effects of valvular lesions and ventricular dysfunction.
Maternal complications depend mainly on functional classification of the mother (NYHA classification is in glossary). NYHA classes I–II have a maternal mortality rate of <1%, whilst NYHA classes III–IV have a maternal mortality rate of 7% or greater2. This is largely determined by the degree of cyanosis, ventricular dysfunction and prior cardiac events, such as pulmonary oedema, arrhythmia, stroke, etc.3 Complications include heart failure, arrhythmia, pulmonary/paradoxical embolism and haemorrhage. The effects on the fetus are marked, with a high incidence of spontaneous abortion, a 30–50% risk of premature delivery and low birth weight. The degree of maternal hypoxaemia is the most important predictor of neonatal outcome; oxygen saturations <85% give only a 12% chance of live birth4. NYHA class IV mothers also have a fetal mortality rate of around 30%. Recurrence risks are generally around 4% but may be up to 50% for patients with 22q deletion, which may not have been recognised previously.
NON-PREGNANCY TREATMENT AND CARE
These women should be under regular, careful supervision of cardiologists in tertiary centres specialising in the care of adults with congenital heart disease. They are monitored:
- Clinically, including oxygen saturation, and Hb estimation
- Functionally, with cardio-pulmonary exercise testing etc.
- By echocardiograph, plus CT or MRI as required
- Regular Holter monitoring for occult arrhythmia
Many will require trans-catheter or surgical interventions and indeed may benefit from new technology as time progresses.
PRE-CONCEPTION ISSUES AND CARE
Ideally, all women should be able to make an informed choice about pregnancy based on both maternal and fetal risks, long-term prognosis and alternative options such as contraception, termination of pregnancy, adoption, surrogacy and IVF. Encourage realistic expectations, and advocate active preparation for pregnancy. This includes:
- Ensuring easy access to multidisciplinary care
- Genetic counselling
- Pre-pregnancy surgery or intervention: e.g. change of valve type; consider risk of surgery versus reduction in pregnancy risk; catheter interventions; ablation of arrhythmia; and optimisation of cardiac function
- Optimal timing of pregnancy (pregnancy at a younger age is lower risk for some complex conditions)
- Avoidance of teratogens (medication may need to be changed prior to pregnancy, e.g. anticoagulants, ACEI, anti-arrhythmics) (see Cardiac Drugs in Appendix 4.3.1)
- Prompt initiation of iron supplementation
- General measures: smoking cessation and folic acid supplementation
- Admission for bed-rest +/− oxygen therapy may be required
- Anaemia should be avoided
- Fetal echocardiography is required as well as regular fetal wellbeing and growth monitoring
- Appropriate timing for delivery is crucial to balance maternal and neonatal morbidity and mortality
- A clear plan of management for labour and delivery should be established in advance, clearly documented and widely available
- Iron deficiency – monitor red cell indices plus Hb, because cyanotic patients can be functionally anaemic even with a ‘normal’ Hb
- Warfarin may not be essential especially for Fontan patients; change to aspirin, or low-molecular-weight heparin
- Stop ACE inhibitors if not done pre-conceptually
- Regular outpatient review, echocardiography +/− Holter monitoring
- Beta-blockers for arrhythmia +/− pacemaker if indicated
- Diuretics for heart failure/breathlessness5
- Oxygen therapy may help severely cyanosed patients, especially if they have a reduced respiratory capacity
- Early booking, with thorough history taking, and immediate referral to a maternal medicine clinic, then liaison with tertiary cardiologist
- Baseline observations, including oxygen saturation
- Maintain Hb levels as above, with additional dietary advice5
- Advise on antibiotic treatment for any dental or other procedures
- Teach the mother to keep a fetal movement chart after 24 weeks
- Prepare parents for potential pre-term/IUGR baby or fetal loss
- Prophylactic (even temporary) pacemaker is considered for some women as pushing is vagotonic
- If normal birth is anticipated consider shortening the second stage
- If there is evidence of aneurysm formation, or major ventricular dysfunction, caesarean section is preferable6
- Epidural use is recommended
- Avoid supine position, especially for Fontan patients, because caval compression restricts pulmonary blood flow
- Invasive arterial +/− venous pressure monitoring may benefit vaginal delivery
- Cyanotic patients may require intravenous hydration, especially if nauseated
- Prescribe antibiotic prophylaxis as indicated
- Intensive care setting is likely5; experienced staff required
- Administer the antibiotics and any other prescribed drugs as above
- Keep the mother in left lateral position throughout the first stage7
- The midwife may well be required to conduct a normal delivery under close medical supervision, with an emphasis on a short second stage, which could require an episiotomy.
- Low dose oxytocin infusion for the third stage.
- Alternatively the role might be to assist with an instrumental/operative delivery, which may well be pre-term
- Otherwise the care is as in Section 4.1
- The neonate may require intensive care in addition to heart screening
- Contraceptive issues are similar to the previous section
- Most require progesterone-only methods
- Close medical supervision is still required after delivery with monitoring for at least 48 hours
- Review medications prior to discharge: restart ACE inhibitors and warfarin; some beta-blockers are excreted less in breast milk
- Systemic ventricular dysfunction may continue or worsen post-partum so careful review pre-discharge is required
- Cardiac follow-up 4–6 weeks post-delivery for almost all patients
- This mother is not for discharge until reviewed by the medical team
- Basic post-operative care is required if post-caesarean section
- Basic observations may need continuation for longer than usual with emphasis on oxygen saturation, blood pressure and fluid balance
- Advise and support specific to the needs of a mother with a baby on a neonatal unit if delivery was pre-term (see Chapter 1)
- Bereavement care if a stillbirth occurred
- The basic care is as in Section 4.1
4.4 Rheumatic and Valvular Heart Disease
EXPLANATION OF CONDITIONS
Rheumatic heart disease is a complication of rheumatic fever in which acute cardiac valve damage (mainly aortic or mitral) arises from immunologic injury in group A haemolytic streptococcal infection (GAS) – see Chapter 12.8. Incidence remains high in developing countries and the UK immigrant population, hence it is re-emerging as a cause of maternal death2. Chronic sequelae include valve stenosis/regurgitation, arrhythmias +/− ventricular dysfunction. Stenosis is the narrowing of a valve restricting forward flow, commonly affecting the mitral, aortic and pulmonary valves. The latter is rarely problematic. Stenosis may be congenital or acquired as with rheumatic fever or SLE3.
Regurgitation/insufficiency/incompetence results from incomplete valve closure that allows regurgitation of blood back to the preceding chamber. It can be congenital or acquired as with rheumatic fever, Marfan’s syndrome or Ehlers-Danlos syndrome.
Prosthetic heart valves are replacements for diseased heart valves. These can be bioprostheses (donor/animal tissue) or mechanical. They are mainly used on the left side and need lifelong anticoagulation; this complicates pregnancy.
COMPLICATIONS
Mitral valve disease: atrial fibrillation, heart failure, endocarditis or thrombosis may develop. Risk of stroke is increased and increased left atrial pressure may cause pulmonary oedema. Mitral regurgitation causes LV volume overload which may impair function.
Aortic valve disease: stenosis restricts the cardiac output increase required in pregnancy and may cause effort restriction, angina or syncope. Symptoms must be taken very seriously as they indicate a high risk of sudden death. Regurgitation is better tolerated but also leads to LV volume overload, myocardial failure and arrhythmia.
Pulmonary valve problems: isolated mild stenosis is common and of little consequence but more severe stenosis may be revealed during pregnancy by increased cardiac output. Pulmonary regurgitation is mainly post-intervention. Residual stenosis and regurgitation are common as part of more complex congenital heart disease.
Ebstein’s anomaly: the tricuspid valve leaflets are displaced into the right ventricle (see Figure 4.3.1b). The valve is regurgitant and malformed. It is associated with other structural cardiac abnormalities. Incidence of arrhythmia is high3.
Mixed disease is common (multiple valves +/− stenosis and regurgitation) complicating care and worsening prognosis.
Infective endocarditis during pregnancy is rare, with an estimated overall incidence of 0.006% (1 per 100 000 pregnancies) and an incidence of 0.5% in patients with known valvular or congenital heart disease. The incidence is higher in drug addicts. Patients with the highest risk for infective endocarditis are those with a prosthetic valve or prosthetic material used for cardiac valve repair, a history of previous infective endocarditis, and some special patients with congenital heart disease. Bacterial infection, especially in pregnancy, of heart valves/structures can initiate systemic or pulmonary embolism, so antibiotic prophylaxis is given within 2–3 hours of unexpected exposure and for predictable bacteraemia.
Antibiotic prophylaxis is no longer routinely advised advisable for valvular disease unless there are other maternal or obstetric indications such as pre-term rupture of membranes or Caesarean section5,6. However, it is probably still advisable for patients with mechanical valves7.
NON-PREGNANCY TREATMENT AND CARE
- Medication might ease symptoms
- Balloon dilatation of valves may be performed safely for non-calcified valves (preferably after first trimester)
- Surgical repair, or replacement, requires cardio pulmonary bypass and is high risk in pregnancy (especially for the fetus) but can be life-saving
Rheumatic Heart Disease
- Can deteriorate with time
- High index of suspicion with newly arrived immigrant women
- Regular cardiologist review
- Earlier surgery if pregnancy is contemplated
Metal Prosthetic Heart Valves
- Patients require long-term anticoagulation and antibiotic prophylaxis as above
PRE-CONCEPTION ISSUES AND CARE
- Expert clinical and echocardiographic assessment of:
- functional status
- previous cardiac events
- ventricular and valvular function
- pulmonary artery pressure
- current medication8
- functional status
- Discussion of risks associated with pregnancy9
- Discuss risks and benefits of anticoagulant therapy
- Pre-pregnancy interventions, with contraceptive cover, to plan and optimise wellbeing for pregnancy10
- Identify if pregnancy is contraindicated, with either pulmonary hypertension or >2 risk factors below:
- reduced LV systolic function (ejection fraction <40%)
- left heart obstruction: aortic or mitral stenosis with valve areas of <1.5 cm or <2.0 cm2, respectively
- past cardiovascular events (heart failure, TIA, stroke)
- reduced functional capacity with a disease score of NYHA class II or higher11 (Box 4.1.1) as the maternal death rate is 30–60%1
- reduced LV systolic function (ejection fraction <40%)
- Decompensation (third trimester risk)15
- Death increases with left heart obstruction or NYHA class II disease16 (Box 4.1.1). Mortality from 0 to 3%4
- Pre-term delivery and IUGR
- Regular appointments at a combined obstetric/cardiology clinic13
- ECG, echocardiography, USS, electrolytes, chest X-ray13 and repeat as indicated
- Diuretics and beta-blockers may be helpful, +/− bed-rest12
- Risk of systemic emboli if atrial fibrillation, so anticoagulation is required, usually with low-molecular-weight heparin4
- Heart rate control with digoxin, beta-blocker, calcium channel blocker or a combination of these19
- Pulmonary oedema – treat with oxygen and diuretics13, consider intervention.
- Balloon dilatation of mitral or aortic valve should be considered in pregnant patients with severe symptoms or systolic pulmonary artery pressure >50 mmHg despite medical therapy4, preferably after the first trimester13
- If surgery is required, careful monitoring of fetal wellbeing is necessary throughout13; increased risk of preterm delivery +/− IUGR13, cardiopulmonary bypass with increased flow, if needed, to respond to fetal distress
- Anaesthetic agents used in the first trimester may have teratogenic effects11
- Conception to week 12: consider the use of monitored and dose adjusted heparin if on >5 mg/day of warfarin; discuss drug teratogenicity versus the risk of valve thrombosis4,12
- Weeks 12–36: generally warfarin therapy is used4
- If labour starts while on oral anticoagulants (OAC), caesarean delivery is indicated4
- Week 36 Hospital: discontinue warfarin; start heparin titrated to a therapeutic activated partial-thromboplastin time, or anti-factor Xa level20 and check weekly.
- Low molecular weight heparin (LMWH) should be replaced by intravenous unfractured heparin (UFH) at least 36 hours before planned delivery. UFH should be continued until 4–6 hours before planned delivery and restarted 4–6 hours after delivery if there are no bleeding complications4
- Valve thrombosis: thrombolytic treatment first line as risks of embolism, bleeding or placental abruption are less than surgery risk
- At/before booking, refer for combined obstetric/cardiology care
- Re-refer promptly if palpitations or breathlessness are reported, because it is difficult to differentiate cardiac decompensation from the physiological symptoms of pregnancy1,8,21
- Social support, and interpreters can improve outcomes1
- Hospital admission for bed-rest, oxygen therapy, saturation monitoring and fluid balance estimation
- Vaginal delivery anticipated and clear plan of care documented including shortened second stage, caesarean section for obstetric indications13
- Antibiotics as indicated12
- Heparin is discontinued for labour and delivery
- ECG, intravenous infusion (IVI) plus invasive monitoring for moderate or severe disease13
- Avoid fluid overload
- High dependency care, with observations of fluid balance, oximetry and electronic fetal monitoring (EFM)13
- TED stockings and early ambulation4
- Avoid supine and lithotomy positions, left lateral position is recommended13
- Avoid active directed pushing12 (heart rate increase might not be tolerated)
- Syntocinon NOT Syntometrine4; uterine massage reduces blood loss13
- Night after delivery – resume warfarin in the absence of bleeding complications 20
- Haemodynamic monitoring >24 h, treat any pulmonary oedema rapidly with 02 diuretic beta-blockers or diamorphine13
- Contraception counselling13, with emphasis on progesterone implants
- Careful fluid balance surveillance as pulmonary oedema is a serious risk
- Gentle mobilization; supportive assistance with baby care to inspire confidence
- Encourage neonatal vitamin K administration
- Liaison with the multidisciplinary team in readiness for discharge
4.5 Marfan’s Syndrome
EXPLANATION OF CONDITION
Marfan’s syndrome was first described in Paris by Bernard Marfan in 18963. It is an autosomal dominant condition on chromosome 153. It is inherited in 50–75% of cases, and also occurs as a spontaneous mutation. Fibrillin (a fine fibre found in connective tissue) production is affected3–5. This connective tissue disorder presents in various body systems: musculoskeletal, ocular, cardiovascular, respiratory and integumentary6.
Typically, there is a disproportionate length of long bones and little subcutaneous fat, presenting as a tall, thin physique with long fingers and toes and hyperflexibility of joints. Further signs of the condition vary considerably between individuals, who may have one or more of:
- Narrow ‘pigeon’ chest3
- Scoliosis3 (curvature of the spine)
- Flat feet3
- Myopia (short-sighted) 2 and lens problems3
- High arched dental palate with overcrowding of teeth
Although the condition affects men and women equally3,7 this appearance may be more exaggerated in men, with some women being undiagnosed8.
The valve between the left chambers of the heart is defective and may be large and floppy, resulting in an abnormal valve motion when the heart beats. In some cases, the valve may leak, creating a heart murmur. Small leaks may not cause any symptoms, but larger ones can result in shortness of breath, fatigue, and palpitations.
Due to faulty connective tissue, the wall of the aorta is weakened and stretches causing aortic dilatation. This increases the risk that the aorta will tear (aortic dissection), or rupture. The rupture causes serious complications of mitral-valve prolapse, aortic-root dilatation or sometimes sudden death5.
COMPLICATIONS
General Complications
- Joint pain and dislocations, due to joint laxity3,7
- Scoliosis (curvature) of the spine and consequent back pain and mobility restrictions2,3,7
- Spontaneous pneumothorax in >10%3
- Bronchiectasis, asthma and emphysema3
- Hernias3,7
- Fatigue3
Cardiac Complications
- Aortic dissection – accounting for 20% of maternal cardiac fatalities8
- Aortic aneurysm formation
- Dilatation of the aortic root can cause the aortic valve to become stretched and leak
- Aortic or mitral valve regurgitation may worsen leading to increased breathlessness, pulmonary oedema or arrhythmias
- New heart murmurs may present
- Cardiomyopathy may develop
- Arrhythmia may occur
NON-PREGNANCY TREATMENT AND CARE
General Treatment
Early diagnosis, meticulous echocardiographic follow-up and multidisciplinary assessment are essential9. Restrictive lifestyle advice and drugs may be necessary, but should be counterbalanced with the need for a child to develop and mature as normally as possible9. Exercise and a healthy, vitamin-rich diet is encouraged, and smoking strongly discouraged as it destroys elastin3.
Cardiac Treatment
- Beta-blockers might be used9 to reduce aortic root dilatation7. If the aortic root is greater than 45 mm, root replacement may be considered, especially if pregnancy is contemplated10
- Following elective aortic root replacement, patients remain at risk for dissection in the residual aorta11
- Increasingly patients may have been on Losartan or other angiotensin receptor blockers (ARB). These are NOT currently viewed as safe in pregnancy.
PRE-CONCEPTION ISSUES AND CARE
- Start advice in adolescence, reinforced by effective contraception
- Genetic counselling as there is a 50% chance the prospective child could inherit the gene3
- the implications for reproductive choices and relationships should be sensitively discussed alongside genetic counselling12
- feelings of guilt about passing on the condition to children, or of being a family member who has been found not to have the condition, have been reported12
- the implications for reproductive choices and relationships should be sensitively discussed alongside genetic counselling12
- There should be access to expert pre-pregnancy care and implications for pregnancy should be discussed
- Echocardiography is essential
- If aortic root <40 mm reassure about the lesser (1%) risk of dissection13,14
- If aortic root >40 mm advise about increased risk of adverse outcome13,14
- If aortic root >45 mm, suggest elective root replacement prior to pregnancy4,13
- If taking ARBs (e.g. Losartan) change to beta-blockers. Otherwise continue beta-blockers. Consider starting beta-blockers if not already on medication.
- Discussion of pregnancy care and possible need for hospitalisation15
- Premature labour and ruptured membranes are common8,17
- Pelvic instability and backache increase
- Care plan devised and updated as delivery may occur at short notice8
- Monitor aortic root diameter with serial echocardiograms
- Consider surgical repair if dilation greatly increasing, but consider gestation and elective delivery by LSCS prior to surgery (association with fetal loss)4,13
- Those with an aortic root diameter of <40 mm historically tolerate pregnancy well, though no evidence for safe diameter 5,14,19,21
- Close monitoring for hypertension which should be treated aggressively8
- Beta-blockers are continued throughout pregnancy, maintain heart rate <110 4,8,21
- Regular monitoring of fetal growth by ultrasound14
- A pre-delivery anaesthetic assessment is advised8,20
- MRI of the pelvis (to detect dural ectasia) may be useful to guide epidural catheter placement8
- Steroids if delivery likely <34 weeks, but this risks fluid retention and cardiac failure, hence diuretics should also be considered22
- Anticoagulants need reviewing
- Aortic-root dilatation may be a risk predictor, but aortic dissection may occur without a clinically significant dilatation5
- Surgery in pregnancy is possible if root dilatation presents14,19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


