Features
Arterial
Atherosclerosis of aorta, carotid, iliofemoral, coronary arteries
Thrombosis of the aorta or axillary, carotid, hepatic, iliofemoral, mesenteric, pancreatic, popliteal, splenic, or subclavian artery
Valve involvement
Valve abnormalities including leaflet thickening, vegetations, nonbacterial thrombotic endocarditis, Libman-Sacks endocarditis
Myocardial involvement
Ventricular hypertrophy, diastolic and systolic dysfunction, myocarditis, heart failure
Coronary artery disease
Embolization or atherosclerosis, angina, myocardial infarction, microvascular damage
Intracavitary thrombi
Peripheral embolization, myocardial microthrombi
Pulmonary hypertension
Pulmonary emboli, pulmonary arterial in situ thrombosis
Cardiovascular complications in APS may be directly related to the presence of autoantibodies or secondary to thrombotic vessel occlusion. These pathological mechanisms produce valvular damage due to the deposition of immune complexes; left ventricular diastolic and systolic dysfunction due to microvessel injury, atherosclerosis of the great vessels and coronary artery disease, myocardial infarction, and endocavitary thrombi; and right heart failure due to pulmonary hypertension. Both valvular abnormalities and coronary artery disease account for more than two-thirds of the cardiac manifestations in APS. Other cardiac manifestations, such as pericarditis and myocarditis, have mild subclinical features and may go undiagnosed in APS patients. Accelerated atherosclerosis is another feature of APS-mediated vascular damage. The pathological process may be mediated by the direct or indirect mechanisms related with the presence of antiphospholipid antibodies [2]. This review will focus on the cardiac presentations in APS patients: the pathological mechanisms, clinical presentation and diagnostic tools, and therapeutic considerations.
11.2 Pathogenesis
APS is a prothrombotic disease caused by antiphospholipid antibodies (aPLs). The term antiphospholipid is a misnomer, because the antibodies itself are directed against plasma proteins, specifically the DmI of the β2GPI molecule [3]. The mechanisms by which this interaction causes the cardiac involvement are multiple and not fully understood. They are related not only to thrombosis [4] but also to the deposition of immune complexes in various sites and the cross-reactivity between antibodies and other self-antigens [5].
11.2.1 Valvular Involvement
Valvular involvement in APS is the most frequent finding. The pathological role has been attributed to the circulating aPL antibodies that may bind the valvular endothelium leading to superficial thrombosis or subendocardial inflammatory infiltration. One possible hypothesis for the genesis of aPL antibodies is the molecular mimicry; in fact, APS may be unleashed by streptococcal infections, and cross-reactivity between the streptococcal M-protein and aPLs has been reported [6] raising the possibility for an infectious origin of APS. In a study seeking to find the β2GPI-related target epitopes recognized by the self-antibodies on the valves, one of the β2GPI-related synthetic peptides was a specific target for anti-β2GPI antibodies deposited on the valves of APS patients [7]. This synthetic peptide was able to displace the anti-β2GPI anti-idiotypic antibodies by a competition assay [7].
The histological analysis on valve specimens in patients with APS revealed that the noninflammatory lesions were characterized by superficial or intravalve fibrin deposits, vascular proliferation, laminar or verrucous superficial thrombosis, intravalve capillary thrombosis, and calcification [6, 8, 9]. Microscopy revealed positive staining for aCL (mainly IgG), for immunoglobulins, and for complement (C1q, C3c, and C4) all appearing along the surface of the leaflets and cusps as a continuous ribbonlike layer with complement deposits being more granular [10]. On the other hand, control valves from aPL-negative patients and control tissue specimens from an APS patient do not demonstrate such deposits [10]. The deposition of aPL antibodies in the subendothelial layer of the valve initiates an inflammatory process [11] with upregulation of markers of endothelial cell activation while the inflammatory exudate was scant. α3β1 integrin, laminin, fibronectin, and a thick layer of collagen IV are present in the deformed valves, while controls did not express the integrin and had only a thin subendothelial band of collagen IV [12].
The two-hit model [13], the most accredited pathophysiologic mechanism relating the presence of aPLs to the clinical features in APS, can be used to explain the valvular damage in APS. Microinjuries in hemodynamically vulnerable sites expose negative phospholipids on the surface of valvular structures or on endothelial cells of intravalvular capillaries [14]. β2GPI changes conformation exposing the DmI to the immune system and is recognized by its antibodies; the resulting complexes cause cell activation and tiny spots of coagulation. Microinjuries, fibrosis, and calcification result in valve thickening and rigidity leading to valvular dysfunction.
A few words should be spent on Libman-Sacks endocarditis. This entity is associated with SLE and, although used interchangeably with APS, is strictly speaking distinct pathological entity. Unlike the bland lesions of APS, Libman-Sacks endocarditis is a true valvulitis replete with inflammatory cell infiltration [15].
11.2.2 Accelerated Atherosclerosis
Accelerated atherosclerosis has been found in APS patients more frequently than in the general population [16]. Data come from retrospective studies and case series. No prospective studies confirm an increased risk in APS. Some have postulated a protective role of aPLs against atherosclerosis [17]; however, most of the literature supports the pathological role of aPLs in the development of premature atherosclerosis. Direct and indirect mechanisms are held responsible about this pathological process. aPLs exert a direct proinflammatory and procoagulant activity on endothelial cells; on the other hand, indirect inflammatory/immune mechanisms are mediated by autoantibody cross-reaction [18, 19]. β2GPI is a major antigenic target for antiphospholipid antibodies.
Other than the abovementioned mechanisms, oxidative stress has a key role in atherogenesis [20]. Oxidized low-density lipoprotein (oxLDL) is the principal lipoprotein found in atherosclerotic lesions, and it colocalizes with β2GPI and immunoreactive CD4 lymphocytes. The oxLDL/β2GPI complexes are internalized by macrophages via IgG anti-β2GPI antibody-mediated phagocytosis [21] to form foam cells [22] leading to the formation of fatty streaks and atheromatous plaques. β2GPI specifically binds to oxLDL, but not to native LDL [23]. oxLDL/β2GPI complexes acting as an autoantigen [24] are closely associated with autoimmune-mediated atherogenesis. IgG anti-oxLDL/β 2GPI-complex autoantibodies and their immune complexes were detected only in SLE/APS patients and in its animal model and were strongly associated with arterial thrombosis [21]. Moreover, IgG autoantibodies against oxLDL/β2GPI were significantly elevated in about 40 % of patients with APS as compared to SLE patients without APS [24, 25]. The immune response triggered by immunization with these autoantigens leads to the progression of atherosclerosis [26]. In contrast, immunization with IgM anti-oxLDL antibodies derived from hyperlipidemic mice reduced the incidence of atherosclerosis [21]. These findings confirm the complexity of the atherogenic mechanisms in APS and a different significance of the classes and subclasses of anti-oxLDL antibodies (IgG and IgM). Other chemo-mediators, such as pattern-recognition receptors (Toll-like receptors and scavenger receptors), cytokines (such as IL-1, IL-6, and TNF-alpha), chemokines, and pentraxins (such as CRP and PTX3), have been correlated with atherogenesis [27].
The whole inflammatory response that derives from the above described pathological mechanisms leads to the enhanced production of mediators that stimulate leukocyte adhesion and recruitment to the vessel wall and proliferation of monocyte/macrophages and of vascular endothelial and smooth muscle cells. The production of matrix-degrading proteases and tissue factor by macrophages eventually leads to plaque rupture and thrombus formation [28].
11.2.3 Ventricular Involvement
The pathogenesis of ventricular dysfunction in APS in the absence of valvular or coronary artery disease is unclear. The histological findings range from microvascular thrombosis to endomyocardial fibrosis [29–31]. Several reports have related the presence of aPLs with otherwise unexplained (by conventional risk factors) ventricular dysfunction especially in young individuals [29, 31–33]. Histological evidence of inflammation, intramyocardial arteriolar immune deposits, and widespread thrombosis, with surrounding areas of microinfarction, has been reported [34–36]. Diastolic dysfunction of the right and left ventricle is a more prominent feature in patients with APS or PAPS compared to systolic dysfunction [37, 38]. Myocardial ischemia mediated by antiphospholipid antibody may slow isometric relaxation and impair left ventricular filling, in the same manner as reported in patients with coronary heart disease. Regional wall motion abnormalities in the left ventricle have been correlated to the presence of high titers of antiphospholipid antibodies [39] and might be related to myocardial necrosis due to microvascular damage or myocardial infarction from coronary artery disease. Moreover, microvascular thrombosis may contribute to myocardial hypertrophy [40]. Finally, endomyocardial fibrosis is a rare cause of myocardial dysfunction in patients with APS [30].
11.2.4 Intracardiac Thrombi
While intracardiac thrombi on the surface of prosthetic or morphologically abnormal native heart valves are often seen in patients with APS, intracardiac thrombi on either structurally normal valve leaflets or unrelated to cardiac valves and involving mural endomyocardium appear to be uncommon.
Although antibodies are thought to contribute to intracardiac thrombus formation, the exact mechanism is still unclear. One of the proposed mechanisms is the disturbance of the physiologic fibrinolysis by aPLs, which, in presence of predisposing factors, brings about thrombus formation on the endocardial surface [41]. Other authors have speculated that disturbances in intracardiac blood flow pattern might contribute to thrombosis [42]. Echocardiographic studies have reported the presence of spontaneous echo contrast in APS patients [43]. Spontaneous echo contrast is caused by reversible red cell agglutination produced by intracardiac stasis rather than by activation of the clotting cascade [44]. How aPLs may contribute to it, is another unexplained issue.
11.2.5 Pulmonary Hypertension
The pathophysiology of the pulmonary hypertension in APS is strongly related to embolism from the veins of the lower limbs, to in situ thrombosis, or rarely to emboli derived from the right heart chambers. Chronic thromboembolic pulmonary hypertension (CTEPH) then builds up over time. Pulmonary embolism alone is not the only actor in the pathophysiology of CTEPH, as the vast majority of the thrombi resolve within a few weeks of the acute event [45]. Although venous thromboembolism (VTE) is the initial event in the vast majority of cases, in situ thrombosis in the pulmonary vasculature may also have an important role, especially during the course of the disease. In situ thrombi are histologically indistinguishable from thromboemboli and thus their role is difficult to be established. On the other hand, a causative role for aPL in the development of CTEPH other than the increased risk for VTE has to be established. One of the proposed mechanisms is an interaction between aPL and endothelial cells on the pulmonary vasculature, leading to vascular remodelling. The whole process may be enhanced by the implication of endothelin-1, a peptide that induces vasoconstriction and stimulates the proliferation of vascular muscle cells, high levels of which have been found in plasma of patients with APS and arterial thrombosis [46].
11.3 Clinical Presentation and Diagnosis
11.3.1 Valvular Involvement
Almost a third of the patients with primary APS present valve abnormalities [47–49]. However, variability in the studied population, APS testing, and different echocardiography techniques (transesophageal echocardiography which is more sensitive than transthoracic echocardiography) account for a big variability in the prevalence of valvulopathy. APS-related valvulopathy is defined as the echocardiographic detection of valvular regurgitation or stenosis in patients with aPLs in the presence of valvular apparatus involvement including valve thickness of more than 3 mm involving the leaflet’s proximal or middle portion and/or irregular nodules on the atrial face of the edge of the mitral valve and/or the vascular face of the aortic valve [1].
In a review [50] of the largest echocardiographic studies including 168 primary APS patients, the prevalence of valvular involvement as assessed with transthoracic echocardiogram was 32–38 % as compared to 0–5 % in the controls. Left-sided valves were most frequently affected, with mitral valve being involved more frequently than the aortic valve [43, 51, 15]. A rationale behind this distinction might be that the left-sided valves are more vulnerable to microinjuries due to stress, jet effect, and turbulence. This suggests that although the initial valve pathology may be simple nodular thrombi especially on the coapting site, the underlying inflammatory process leads to fibrosis [52]. In general, tricuspid valve seems to be seldom affected [47]. Subvalvular thickening and chordal apparatus involvement is also rare [53]. Heart valve lesions include irregular valve thickening that may evolve to vegetations and rarely lead to clinically significant valvular dysfunction (Fig. 11.1). Peculiarities of the nodular abnormalities resulting from thrombi at the coapting site of the leaflets are the focal localization and symmetry [52]. The extent of fibrosis may determine the degree of valve thickening as well as valve retraction or mobility. Mitral valve thickness can increase to up to 2.5 times that of controls [54].
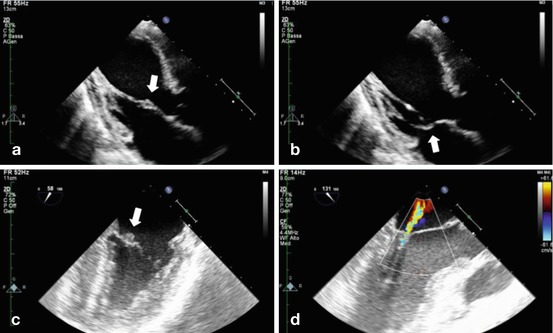

Fig. 11.1
(a, b) TTE showing thickening of the anterior mitral valve leaflet on its midportion (arrow) in a primary APS patient; chordal apparatus is not involved. (c) TEE showing typical vegetation on the atrial surface (arrow) of the mitral valve. (d) The same patient (as in c) had mild mitral insufficiency due to the valvular affection (Source: Echocardiography Laboratory, Cardiology Clinic, Padua University Hospital)
Vegetations are seen less often but might be present in up to 40 % of the patients with aPLs as detected by transesophageal echocardiography [47] and are usually solitary but may be multiple. They present an irregular shape and are predominantly thrombotic but can be inflammatory or mixed, may be mobile or not, and change in appearance, resolve, or reappear over time [52, 55, 56]. Vegetations appear generally on the atrial surface of the mitral valve, while aortic valve vegetations have been described on both the ventricular and the vascular surface of the valve [57].
Valvular involvement is generally subclinical and leads to hemodynamically significant valvular dysfunction only in 3–5 % of patients [58, 51]. The valvular dysfunction results from an improper closure mechanism caused by fibrosis/thickening, valve deformity, and nodular vegetations, resulting in regurgitation. Valvular stenosis is seldom encountered in primary APS patients [43, 59].
Valvular abnormalities may be a risk factor for stroke, particularly with primary APS [60]. In fact, the presence of both stroke and valvulopathy was found in 77.4 % of all patients in one study [59], while thromboembolism in coronary arteries occurs less frequently (20–25 %) [47].
Considering echocardiographic studies, it is still unclear whether patients with primary APS have a higher prevalence of valvular abnormalities with respect to patient with SLE with or without associated aPL. Several studies using Doppler echocardiography have shown a higher prevalence of valve defects in patients with primary APS and SLE with aPL than in patients without these antibodies [51, 49, 43]. In fact, in large echocardiography study of 200 patients with SLE [61], aPL antibody positivity (IgG subtype) was most frequently associated with mitral valve nodules and the presence of moderate to severe mitral regurgitation. No correlation between aPL antibodies and aortic valve involvement was found [61]. The incidence of valve abnormalities in primary APS was as high as 82 % in one study [43]. In the same study, the authors confirmed that mitral valve thickening is most commonly found (63 %), followed by aortic and tricuspid valve thickening (32 and 8 %, respectively) [43]. Valve involvement in secondary APS has similar characteristics [15]. Localized thickening is generally confined to the base or midportion of the affected leaflet [62]. Vegetations, occurring in 10–40 % of the patients [47], are irregular in shape, broad based, and immobile [62, 39]. Lesions are also typically located on the atrial surface of the mitral valve and the vascular side of the aortic valve, with a predilection for the leaflet base [62].
11.3.2 Differential Diagnosis
Due to the nature and characteristics of the valvular lesions in APS, it might become clinically and therapeutically important to distinguish them from infective endocarditis (Table 11.2) and rheumatic disease (Table 11.3).
Table 11.2
Differential diagnosis with infective endocarditis (see text for a detailed description)
Echocardiographic feature | IE | APS |
|---|---|---|
Vegetation mobility | Mobile (pedunculated) | Mobile or immobile (broad based) |
Tissue destruction | Present | Absent |
Vegetation location | Usually near leaflet-free margins | Base to mid (especially SAPS) or free margin (especially PAPS) |
Lesion number | Vegetations usually solitary | Single or multiple (e.g., kissing vegetations) |
Echogenicity | Usually homogeneous | Usually heterogeneous |
Symmetry | Asymmetric | Usually symmetric |
Leaflet thickening | Absent | Often present |
Mitral valve surface | Atrial | Atrial; occasionally ventricular |
Aortic valve surface | Ventricular | Ventricular or vascular |
Table 11.3
Differential diagnosis with rheumatic heart disease (see text for a detailed description)
Echocardiographic feature | Rheumatic valve disease | APS |
|---|---|---|
Valves involved | Typically the mitral | Both may be involved, usually the mitral |
Vegetations | Absent | Present |
Increased leaflet thickness | Free margins toward base | Base to midportion |
Chordal lesions | Frequent | Rare |
Calcifications | Present | Absent |
Leaflet fusion | Present | Absent |
Valve stenosis | Frequent | No |
Noteworthy, aPLs may be present in some infectious diseases and even infective endocarditis [63]. On the other hand, culture-negative patients presenting with fever, and aPLs, can pose significant diagnostic challenges. Echocardiography becomes an important tool for the differential diagnosis as valvular lesions have discriminating echocardiographic features.
Vegetations of infective endocarditis tend to be solitary, asymmetric, of uniform echogenicity, and highly mobile [64]. In contrast to the vegetations of APS, they typically affect the low-pressure sides of the valves; they are typically found on the atrial surface of the mitral valve and on the ventricular side of the aortic valve. Furthermore, the adjoining leaflet or cusp is usually damaged in infective endocarditis. Anatomically, unlike the sterile vegetations of APS, infective endocarditis is characterized by a combination of vegetations, destructive lesions, and abscess formation, resulting in severe early complications of the disease like chordal rupture and valvular destruction leading to acute heart failure [64].
Valvular involvement may be encountered in both rheumatic heart disease and APS, but their echocardiographic characteristics are different [65]. Moreover, other characteristics of valvular involvement in rheumatic heart disease, and that are unlikely in APS, are thickening of the subvalvular apparatus chordal structures, leaflet restrictions, commissure fusion, and leaflet and annular calcification; all these features are commonly found together especially in the advanced stages of the disease. In addition, unlike the valvular dysfunction in APS, rheumatic valvular disease usually progresses to stenosis rather than regurgitation. Another peculiar distinction is that unlike APS, leaflet thickening in rheumatic valvular disease generally progresses from the free margin towards the leaflet base. The chordae and subvalvular apparatus are typically thickened, fused, and foreshortened in rheumatic heart disease, while in APS, nodules may be seen attached to otherwise normal chordal structures [15].
Papillary fibroelastomas, a rarely encountered cardiac tumor, can also enter differential diagnosis with APS vegetations. These are typically solitary highly mobile vegetations, with a characteristic shimmering or vibratory appearance, and are typically located on virtually all endocardial surfaces [66].
11.3.3 Accelerated Atherosclerosis
Although premature or accelerated atherosclerosis has been associated with the presence of aPLs and APS [16], atherosclerosis in patients with primary APS has not been widely assessed. Existing studies are scarce and have dealt with a relatively small number of patients. Most studies have included both patients with primary and secondary APS. A higher number of carotid plaques have been linked to SLE-associated APS in comparison to primary APS [28]. In addition, secondary APS patients also had a higher prevalence of traditional risk factors for CVD [67].
Despite being frequently of subclinical entity, accelerated atherosclerosis is an important concern in primary APS because it may become clinically relevant rapidly in the young individuals [68, 2] and contributes to important cardiovascular morbidity (Fig. 11.2). In fact, premature atherosclerosis is a possibility in primary APS patients starting in their fourth decade of life [69], and most importantly intimal-medial thickness has been linked to a 3-fold higher risk for stroke in patients with primary APS patients as compared to patients without intimal-medial thickness [68].
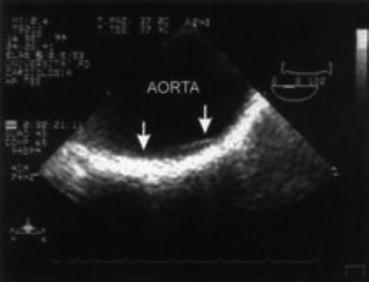

Fig. 11.2
Atherosclerotic lesion (arrows) on the abdominal aorta of a young patient with primary APS
Traditional (Framingham) risk factors for cardiovascular disease, such as hyperlipidemia, diabetes mellitus, smoking, obesity, arterial hypertension, and sedentary lifestyle, do not account for the increased risk for accelerated atherosclerosis in APS patients as their prevalence in APS patients is no different from that of the general population [19]. The increased risk might be explained by considering additional risk factors such as inflammation and autoimmunity (as described in the Sect. 11.2 of this chapter). Carotid arteries are most commonly affected, but peripheral artery disease evolving to claudication is not uncommon (Fig. 11.2).
Few specific studies aimed at the detection of atherosclerosis in APS have been performed and current information comes from cohorts assessing other cardiovascular manifestations of APS. Imaging techniques are sensitive enough to detect subclinical atherosclerosis by assessing early endothelial dysfunction, abnormalities of circulation, or atherosclerotic plaques [2]. Carotid intimal-medial thickness (IMT) is considered an early sensitive marker of generalized atherosclerosis [70]. In one study, primary APS patients had significantly higher carotid intimal-medial thickness and reduced internal lumen diameter as compared to controls [68]. The authors [68] suggest that atherosclerosis in patients with primary APS develops primarily by generalized IMT rather than by atherosclerotic plaques. In the presence of continuous stressing factors, one of them being the persistence of aPLs, these lesions progress to manifest atherosclerosis [71].
Imaging studies have confirmed an increased incidence of early endothelial dysfunction and increased common carotid intimal-medial thickness in patients with APS [2]. An increased incidence of premature atherosclerosis was found in premenopausal women with APS undergoing vascular ultrasound; an increased prevalence of carotid and femoral plaques not related to any other predictors of atherosclerosis tested including age, lipid levels, or cumulative corticosteroid use was found [2]. Overall, patients with primary APS have a high prevalence of increased carotid artery intimal-medial thickness and lumen diameter decrease, with or without clinically relevant atherosclerosis. It may be easily detected with Doppler carotid ultrasound. Its detection may have important prognostic and therapeutic values, as this condition is related with an increased risk for stroke.
11.3.4 Coronary Artery Disease and Myocardial Infarction
Patients with APS have an increased risk of developing coronary artery disease. The mechanism of epicardial coronary artery involvement in APS is related to the risk of accelerated atherosclerosis; however, myocardial infarction can occur in patients with normal coronary arteries, due to thromboembolism or microvascular thrombosis (Fig. 11.3). Unlike the classic coronary syndrome, myocardial infarction in APS manifests in the young patients. Almost all the patients have structurally normal coronary arteries, while coronary artery abnormalities are seldom encountered and might be merely incidental findings.
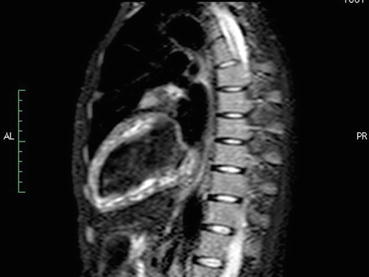

Fig. 11.3
STIR sequence images on a cardiac magnetic resonance in a patient with primary APS and diffuse myocardial ischemia, as documented by the diffuse subendocardial LGE, due to microvascular involvement
In one of the largest cohorts of APS patients [72], myocardial infarction was a presenting manifestation in 2.8 % of the patients and appeared during the follow-up in 5.5 % of the cohort. However, the risk of myocardial infarction associated with aPLs in the general population remains unclear. There is still controversy as whether aCLs and aβ2GPI antibodies are associated with an increased risk of myocardial infarction and coronary artery disease in different settings of APS [73, 74].
Some authors have suggested a positive correlation between aβ2GPI and myocardial infarction in the setting of secondary APS. In a study on patients with cardiovascular disease, less than 2.6 % of patients were positive for aCLs, as compared to 36 % testing positive for anti-β2GPI [75]. The relative infrequency of aCLs in patients with CAD and APS without underlying autoimmune disease was confirmed in another prospective study [76]. The presence of aCL antibodies is also associated with typical chest pain and significant coronary artery stenosis on angiography and is predictive of myocardial infarction [49]. Despite the positive association with adverse events and outcomes especially in young patients [76], angioplasties [77], and coronary artery bypass [78] that has been reported, negative associations have also been found [79].
On the other hand, one prospective study evaluated the association of aPLs with CAD severity and adverse outcomes in patients with acute coronary syndrome [76]. The authors found that aPLs (anti-β2GPI and anti-oxLDL/β2GPI antibodies) occurred in patients with acute coronary syndromes and were strongly associated with the severity of CAD and adverse outcomes. Anti-β2GPI antibodies were found to be a significant risk factor for MI in young premenopausal women independent of other risk factors [80]. After adjusting for traditional risk factors, IgM anti-β2GPI was a stronger risk factor for MI than IgG.
The association of aCL and aβ2GPI antibodies with the risk of recurrent cardiac events in postinfarction patients was assessed in a large trial [74]. High IgG and low IgM aCL antibody titers were independent risk factors, conferring a 3-fold higher risk of recurrent cardiac events. No significant association of the aβ2GPI antibodies with recurrent cardiac events was found.
Lupus anticoagulant (LA) has also been related to myocardial infarction. The odds ratio for myocardial infarction in patients testing positive for LA was 5.3 and increased to 21.6 in women who used oral contraceptives and 33.7 in those who smoked [79]. Neither aCL nor aβ2GPI antibodies affected the risk of myocardial infarction [79].
Because of the nature of the myocardial damage in APS patients (mainly due to microvessel involvement rather than conventional CAD), the condition might go unrecognized. Thus, the real prevalence of myocardial ischemia is not known. In one study, the prevalence of unrecognized myocardial scarring detected by cardiac magnetic resonance was surprisingly high [81]. Despite the low pretest probability for coronary artery disease and myocardial ischemia, late gadolinium enhancement (LGE) displayed a typical pattern of myocardial ischemia in 11 % of the patients with APS as opposed to 3.7 % in control subjects. The levels of aβ2GPI antibodies had a tendency to correlate with the presence of myocardial ischemia detected by LGE.
The discrepancy in the reported data comes from the differences in the study design, differing definitions of aPL positivity, and differences in the cohorts of patients studied. In fact, most studies searched for aPLs in myocardial infarction survivors rather than vice versa. Most studies searched for aCL correlation with myocardial ischemia; however, true high-risk APS patients are those with aβ2GPI positivity in a triple laboratory positivity setting [82]. However, overall, there seems to be an increased risk of myocardial infarction and recurrent events in patients with aPLs and that APS patients have an increased risk of developing ischemic heart disease.
11.3.5 Abnormalities of Ventricular Function
Limited data are available regarding abnormalities of ventricular function in APS. The prevalence of systolic and diastolic dysfunction in APS has been examined in very small studies. From the available data, there seems to be a distinction in ventricular function involvement in patients with primary and secondary APS. Patients with primary APS appear to have more diastolic dysfunction, while patients with SLE and associated APS have more systolic dysfunction [37, 83]. This might be related to the physiopathology of the diseases; SLE probably has a higher autoimmune burden and thus affects systolic function by other mechanisms rather than only autoimmune involvement of the myocardium [83]. However, APS itself seems to contribute to increased biventricular impairment than SLE itself. Thus, it seems that SLE may lead to subclinical systolic dysfunction, whereas the presence of APS (primary or secondary) possibly contributes to diastolic dysfunction [37]. Another peculiarity of primary APS seems to be the organic selectivity. In one cross-sectional study of patients with SLE and APS [38], the parameters of right ventricular diastolic function were more pronounced than the impairment of the respective left ventricular parameters in APS patients (systemic hypertension and valvular disease patients were excluded). Echocardiographic parameters reflecting diastolic dysfunction were associated with high titers of aCL, APS, disease duration, and pulmonary hypertension. There was gradation of severity, with more severe impairment in patients with primary APS followed by the group with secondary APS. One proposed explanation was that microvascular disease might have more effect on the right ventricle, which has a substantially smaller mass than the left ventricle. Furthermore, other factors such as pulmonary hypertension, frequently associated with APS (see below the dedicated section), may also explain the predominantly right ventricular diastolic impairment. In that context, pulmonary hypertension and primary APS were the strongest independent predictors of a prolonged duration time.
The effect of aPLs (in the absence of other confounding factors) in left ventricular size and function remains uncertain, due to the small number of patients studied. In a prospective study of 18 primary APS patients without clinically evident cardiac disease, the authors report a prolonged isovolumetric relaxation time, abnormal left ventricle early filling pattern, and decreased myocardial lengthening rate compared with age- and sex-matched healthy controls [35]. Others confirmed the impaired diastolic filling in primary APS versus age- and sex-matched healthy controls [84]. There are other reports showing that aPLs are not associated with significant abnormalities of myocardial structure, systolic function, or hemodynamics [61].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


