14.1 Iron Deficiency Anaemia
EXPLANATION OF CONDITION
Iron deficiency is the commonest cause of anaemia amongst women of child-bearing age and in particular pregnant women (51%) worldwide2.
Symptoms vary from mild tiredness to potentially hazardous palpitations, breathlessness or symptoms of high output cardiac failure. In humans, mineral iron is present in all cells and carries oxygen to the tissues from the lungs in the form of haemoglobin (Hb), facilitates oxygen use in muscles as myoglobin, and also in cytochromes within cells for enzyme reactions in tissues.
Women have approximately 2.3 g total body iron of which most (80%) is found in the red blood cell mass as haemoglobin (Hb). Total body iron is determined by intake, loss and storage of this mineral. Any iron not in use is stored as the soluble protein complex ferritin, present primarily in the liver, bone marrow, spleen and skeletal muscle. Normal absorption mechanisms in the gastrointestinal system of the body are required to maintain the balance between functional iron (Hb) and stored iron levels. The body is able to absorb 1–2 mg iron daily from the diet, with the aid of absorption enhancers in the diet and a satisfactory rate of red blood cell production. The main factor controlling iron absorption is the amount of iron stored in the body and the type of iron in one’s diet3.
Anaemia results in a reduction in the oxygen-carrying capacity of the blood. Iron deficiency anaemia is defined by a low serum ferritin concentration of <30 micrograms/l and haemoglobin <11.0, 10.5 and 10.5 g/dl in the first, second and third trimesters respectively2,4,5. Red blood cells are microcytic (small) and hypochromic (pale) on microscopic examination (see Figures 14.1.1 and 14.1.2). Iron deficiency anaemia arises from an increase in iron requirements or inadequate iron absorption.
Figure 14.1.1 The development of iron deficiency anaemia. Reticuloendothelial (macrophage) stores are lost completely before anaemia develops (Hoffbrand, 2011). MCH, mean corpuscular haemoglobin. MCV, mean corpuscular volume.
This figure is downloadable from the book companion website at www.wiley.com/go/robson
Figure 14.1.2 The peripheral blood film in severe iron deficiency anaemia. The cells are microlytic (small) and hypochromic (pale) with occasional target cells (Hoffbrand, 2011).
This figure is downloadable from the book companion website at www.wiley.com/go/robson
Iron requirements are increased to deal with:
- Growth
- Menstruation
- Blood loss/donation
- Pregnancy
- Haemolytic disorders
- Drugs that cause haemolysis (e.g. antiretrovirals)
- Genitourinary tract infections
- Hookworm infestation
Anaemia caused by inadequate iron absorption occurs from:
- Diet low in haem iron
- Malabsorption
- Gastric surgery
- Malaria infection resulting in poor use of dietary iron
The amount of functional iron in the body and the concentration of the iron-containing protein Hb in circulating red blood cells are measured by two simple blood tests, Hb and haematocrit and ferritin concentration.
Haemoglobin Concentration and Haematocrit
Haemoglobin and haematocrit (HCT) are both late indicators of anaemia. Haematocrit indicates the proportion of whole blood occupied by the red blood cells, and falls only after the Hb concentration has also fallen. Mean cell volume (MCV) is also important as it falls in iron deficiency, but needs further testing to distinguish it from other causes of microcytosis (see Section 14.6 Thalassaemia).
Serum Ferritin Concentration
Serum ferritin concentration is an early indicator of the status of iron stores and the most specific indicator of depleted iron stores routinely available. A serum ferritin concentration of ≤30 micrograms/l confirms iron deficiency among women who test positive for anaemia on the basis of Hb concentration or haematocrit6. Serum ferritin can be raised in infection and may need repeating for a definitive diagnosis of iron deficiency. Caution is advised in interpretation of normal levels in pregnancy since a low normal level (30–50 micrograms/l) may still indicate iron deficiency. Use of other tests should be confirmed with local laboratories.
COMPLICATIONS
Iron deficiency can interfere with vital body functions leading to morbidity and mortality including:
- Palpitations
- Tiredness
- Irritability
- Depression
- Breathlessness
- Poor memory
- Muscle aches
- Poor appetite
- Cardiac failure
- Increased vulnerability if small amounts of blood are lost
NON-PREGNANCY TREATMENT AND CARE
Encourage iron-rich foods in the diet and treat with iron supplementation 60–120 mg/day for 4 weeks7. If no response to treatment, investigate using other tests: reticulocyte count and serum ferritin concentration. Conditions such as sickle cell trait or thalassaemia minor in women of African, Mediterranean or Southeast Asian ancestry cause mild anaemia unresponsive to iron therapy. The normal Hb level for this group can be as low as 10 g/dl. However, the lowest normal Hb in healthy non-pregnant women is defined as 12.0 g/dl2. Iron absorption can be increased in a vegetarian diet by careful planning of meals to include other sources of iron and enhancers of iron absorption8.
PRE-CONCEPTION ISSUES AND CARE
A non-pregnant woman of reproductive age has an average iron requirement of 1.3 mg/day. This increases when pregnant by an extra requirement of 3.0 mg/day mainly for increases in maternal red cell mass, placental and fetal growth, blood loss at delivery, physiological intestinal blood loss and menstruating loss over the child-bearing years. A further iron requirement of 6–8 mg/day occurs after 32 weeks’ gestation.
Provide culture-specific dietary advice with information on iron body stores and avoidance of absorption inhibitors, e.g. tea, bread and chapatti. A reliable system for investigation of anaemia and monitoring of response to treatment is paramount2.
- Prevention of anaemia by early recognition of iron deficiency in those at risk is paramount
- Anaemia in the third trimester increases the risk of poor recovery from blood loss at birth, as well as tachycardia, shortness of breath and maternal exhaustion
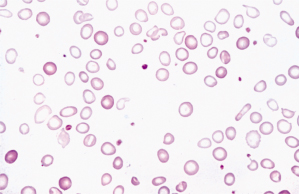
- Parenteral iron, intravenous or intramuscular using the z-track technique (Figure 14.1.3), is used in cases of non-compliance or malabsorption, but contraindicated in cases of allergy
- Be aware of potential skin staining when administering iron, if the z-track technique is not used (see Figure 14.1.4)
- A rise in reticulocyte counts and Hb of 0.8 g/dl/week occurs 5–10 days after starting oral iron treatment9; the increase in Hb is similar for parenteral iron10
- Randomised controlled trials have been inconclusive on the effect of universal iron supplementation in pregnancy versus adverse maternal and fetal outcomes11
- Ensuring normal Hb and adequate iron stores is part of promoting higher likelihood of normality
Figure 14.1.3 Z-track technique for administration of iron to prevent iron staining, as in Figure 14.1.4.
This figure is downloadable from the book companion website at www.wiley.com/go/robson
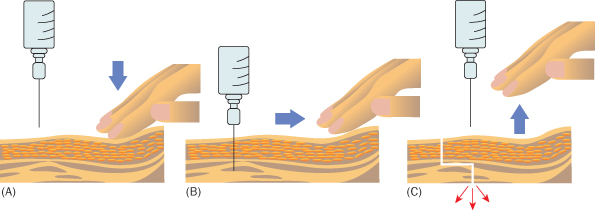
Figure 14.1.4 Iron-stained skin of a pregnant woman (© C. Oppenheimer).
This figure is downloadable from the book companion website at www.wiley.com/go/robson
- Assess cause of anaemia by adequate dietary and medical history and appropriate testing. See algorithm for management (Figure 14.1.5)
- Prescribe ferrous sulfate 200 mg 2–3 times daily, or a proprietary combined iron and folate tablet with a higher elemental iron content until Hb normalises and thereafter to replenish stores. Advise to take iron preparations on ‘an empty stomach’ with orange or apple juice
- If required, im or iv dose is calculated according to iron deficit and body weight
- Administer iron dextran or sucrose iv in 0.9% sodium chloride infusion as total or divided doses
- Consider blood transfusion only if severe anaemia in a situation with a high risk of blood loss (blood products and transfusion are outlined in Appendix 14.1.1)
Figure 14.1.5 Protocol* for community management of anaemia in pregnancy.
* Clinical protocol used for University Hospitals of Leicester, adapted and used with permission. This figure is downloadable from the book companion website at www.wiley.com/go/robson
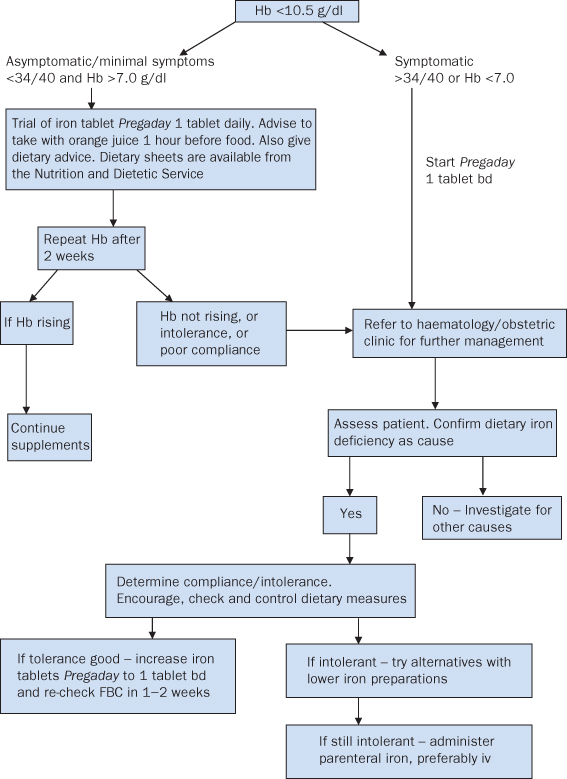
- Severe/chronically anaemic women to be booked at consultant unit
- Encourage consumption of iron-rich foods with orange juice to enhance iron absorption, and provide information on nutrition in pregnancy12
- Be aware that inhibitors of iron absorption include polyphenols (in certain vegetables), tannins (in tea), phytates (in bran) and calcium (in dairy products)
- Screen all women at booking visit and at 28 weeks’ gestation13 and ensure the result is acted upon
- Women with known anaemia need testing at each antenatal visit
- Ensure anaphylactic emergency treatment is available during parenteral iron administration
- Treat selectively with iron1,7 and folate preparations14 and if needed reduce gastrointestinal complaints15
- Maternal exhaustion
- Exacerbation of anaemia by excessive blood loss:
- multiple birth
- prolonged labour
- instrumental delivery
- caesarean section
- grand multiparity
- multiple birth
- Shortness of breath
- Tachycardia
- Group and save serum on admission to labour
- Assess risk factors for excessive blood loss
- Care in consultant-led unit
- Active third stage of labour – syntometrine and IVI of oxytocin
- Await FBC results before advocating eating and drinking in labour
- Vigilant monitoring of labour progress
- Prompt referral to obstetrician if slow progress develops
- Avoid directed pushing where possible
- All perineal trauma to be sutured
- Mother is at risk of:
- postpartum haemorrhage
- infection
- poor wound healing
- postnatal depression
- lethargy
- breast-feeding difficulties
- postpartum haemorrhage
- Mother requires return of Hb to normal level before planning further pregnancies
- Fetus obtains iron from placental transfer regardless of maternal iron stores, hence is unlikely to be anaemic
- Potential for IUGR or pre-term neonate with associated problems16,17
- Reassure mother that the baby is unlikely to be anaemic
- Continue the maintenance dose of oral iron for up to 3 months postpartum to replenish stores
- See algorithm for management (Figure 14.1.5)
- Be alert for signs of postpartum haemorrhage, infection and side effects of iron supplementation
- Postnatal assessment – FBC to identify any extra requirements
- Promote breast-feeding realistically with options to rest, e.g. express breast milk so that baby can be fed by other family members
- Consider social circumstances and use support such as Home Start, family and friends for basic housework
- Reassure mother that baby unlikely to be anaemic.
- Advise an iron-rich diet to improve iron stores (see Patient Organisation page for link to diet sheets)
- Be alert for signs of postnatal depression and continue postnatal visiting if indicated
- Contraceptive advice to ensure adequate spacing of pregnancies
14.2 Megaloblastic Anaemia
EXPLANATION OF CONDITION
Megaloblastic anaemia is an acquired condition characterised by macrocytosis – the MCV of the red blood cells (erythrocytes) is above the normal range of 80–95 femtolitres (fl). It is called megaloblastic because the developing red blood cells in the bone marrow are larger than normal and have immature nuclei. A full blood count may reveal an increased number of immature red blood cells (megaloblasts) in the blood, reduced platelets and haemoglobin levels unresponsive to treatment with iron supplements, as well as the raised MCV.
Megaloblastic anaemia is usually caused by deficiency of folic acid or vitamin B12 (cyanocobalamin), but more rarely may be drug-induced or associated with myelodysplastic syndrome. It is also possible to see a non-megaloblastic macrocytic picture in liver disease, hypothyroidism and alcoholism.
In pregnancy a relative macrocytosis is common and normal.
Folate deficiency is associated with nutritional and socio-economic status2 and may cause complications in pregnancy. Megaloblastic anaemia develops insidiously following:
- Poor dietary folate intake
- Excessive alcohol consumption3,4
- Increased cell turnover due to:
- pregnancy (demands of mother and fetus)
- chronic haemolytic anaemia (e.g. sickle cell anaemia, hereditary spherocytosis)
- chronic inflammatory conditions
- pregnancy (demands of mother and fetus)
- Renal loss
- chronic renal failure
- dialysis
- chronic renal failure
- Malabsorption disorders, e.g. gluten-induced enteropathy (coeliac disease)
- Drug-induced
- some anticonvulsants
- sulfasalazine
- methotrexate
- some anticonvulsants
Mainly stored in the liver, cellular folates help to build DNA and protein in all tissues. This includes those required for growth of the fetus, placenta, maternal red cell mass and uterine development in pregnancy. Folate is found in beans, rice and green vegetables and some food stuffs have folic acid supplemented.
Vitamin B12 deficiency is caused by veganism or poor quality diet, pernicious anaemia, gastrectomy or ileal resection (as occurs with Crohn’s disease).
COMPLICATIONS
The consequences of true megaloblastic anaemia include:
- Pallor and jaundice
- Increasingly severe anaemia
- Heart failure
- Pancytopenia (low white cell and platelet counts)
Other complications of vitamin B12 deficiency include:
- Neuropathy involving peripheral nerves and spinal cord
- Psychiatric disturbances
- Visual disturbance
- Fetal neural tube defects
Both deficiencies can cause epithelial disturbance, e.g. the smooth painful tongue (glossitis), and, if low serum homocysteine levels are present (part of this metabolic pathway), arterial obstruction and venous thrombosis.
NON-PREGNANCY TREATMENT AND CARE
Advise a diet rich in vitamin B12 and folate, such as cheese, cereals, leafy green vegetables, fortified cereals, fruit and egg yolks. The recommended daily intake of folate is 3 micrograms/kg of body weight for non-pregnant and non-lactating women.
A daily intake of 5 mg folic acid is recommended for women with hereditary haemolytic disorders, epileptics on anticonvulsants and women of reproductive age with a family history of neural tube defects who are planning a pregnancy4–6.
PRE-CONCEPTION ISSUES AND CARE
Estimated dietary requirements of folates in pregnancy are 100–600 micrograms/day with an average daily intake of 237 micrograms/day7,8.
An association exists between periconceptual folic acid deficiency and neural tube defect, cleft lip and cleft palate in the fetus5, hence the recommended folic acid supplementation of 400 micrograms/day for the first 3 months pre-conceptually and throughout the first trimester4,9.
A daily oral supplementation of 5 mg folic acid is recommended for those with a previously affected fetus and family history of neural tube defects and to epileptics4,10.
Other factors such as genetic factors, pre-conceptual diabetes and first trimester hyperglycaemia and drugs such as sodium valproate used for epilepsy also contribute to the development of megaloblastic anaemia. Also relevant are the increased demands of pregnancy, multiple pregnancy, grand multiparity or frequent pregnancies3 which may exacerbate other factors or in extreme cases precipitate megaloblastic anaemia.
- Cervical dysplasia
- Loss of appetite and maternal weight loss
- Glossitis
- Increased risk of neural tube defects
- Increased risk of fetal cleft palate
- IUGR
- Full blood count; assess serum and red cell folate levels9
- Mild/moderate anaemia – synthetic oral folic acid 5–10 mg/day
- Folic acid oral or im supplement may be prescribed
- Vigilant screening for congenital abnormalities – ultrasound scan
- Further investigations for associated conditions if megaloblastic anaemia presents for the first time in pregnancy4, but ensure this is true megaloblastic anaemia; obstetrician may need to liaise with physician/haematologist
- Infection screening may be indicated
- IUGR – consider serial ultrasound scanning
- NICE recommend routine supplementation of oral folic acid 400 micrograms/day prior to pregnancy and in the first trimester9
- Midwife to take thorough booking history to identify any current treatment and co-existing conditions that may influence megaloblastic anaemia, e.g. haemoglobinopathies, dietary restriction
- All such mothers should be booked at a consultant unit
- Weigh at each antenatal appointment if there is any suggestion of weight loss or loss of appetite
- Conduct a nutritional assessment referring to a dietician if indicated
- Promote a folate-rich diet and taking of prescribed supplements
- Encourage attendance at antenatal parent education with an emphasis on food preparation
- Assess for signs of infection and refer for treatment promptly
- Severe anaemia is treated with folic acid 5–10 mg/day im oral iron (and blood transfusion as a last resort)
- Counselling about the increased risks of congenital anomalies is advised if confirmed folate deficiency in the first trimester
- Be alert for IUGR and refer as appropriate
- Be alert for APH and advise mother to seek prompt help if symptoms present, including giving of emergency telephone numbers
- Prepare mother for the possibility of blood transfusion in labour
- Increased risk of prematurity and low birth weight in severe cases
- Theoretical risk of postpartum haemorrhage in extreme B12/folate deficiency; however, this is rare.
- Maternal tiredness is more likely at the onset of labour
- If symptomatic anaemia presents, repeat full blood count with cross-matched blood available if needed
- No specific recommendations in labour and birth if blood count is stable
- Encourage mobilisation and maintain hydration to combat tiredness
- Actively manage third-stage labour to reduce blood loss at birth, especially if symptoms of anaemia persist
- Alert neonatal team if premature birth is imminent
- Human milk has a folate content of 5 micrograms/dl, therefore red cell folate levels are further depleted in lactating women
- Women with folate deficiency diagnosed prenatally should continue supplementation for several weeks postpartum
- Adequate inter-pregnancy interval is advised to encourage woman to fully recover and maintain good folate reserves
- Indices of folate metabolism return to pre-pregnant values within 6 weeks of delivery
- Haematological follow-up may be needed
- Continue to promote a diet rich in folates
- Full blood count to determine haemoglobin status, red cell folate concentrations, reticulocyte count and blood film recommended if symptomatic anaemia occurs
- Provide contraceptive advice and consider further discussion with women suffering from epilepsy and malabsorption disorders
- Continuation of folate supplementation advised for those with hereditary haemolytic disorders and epilepsy
14.3 Disseminated Intravascular Coagulation
EXPLANATION OF CONDITION
Disseminated intravascular coagulation (DIC), also known as consumptive coagulopathy, is an acquired disorder of haemostasis, which often heralds the onset of multi-organ failure2.
Underlying causes3:
- Infection – especially Escherichia coli, Neisseria meningitidis, Streptococcus pneumoniae and malaria
- Cancer – especially lungs, pancreas3, gynaecological4
- Trauma, burns, surgery and snake bite5
- Pregnancy – especially:
- placental abruption
- major haemorrhage
- pre-eclampsia
- retained dead fetus or placenta
- amniotic fluid embolism3
- placental abruption
Endothelial damage arising from one of the above results in thromboplastins being released from the damaged cells6, triggering the extrinsic pathway to initiate a coagulation cascade3. With DIC, the tissue damage is so severe that blood clotting occurs at the original site and throughout the vascular tree, hence the term disseminated intravascular coagulation. This process consumes large quantities of fibrinogen, thrombocytes (platelets) and clotting factors V and VIII3. The micro-thrombi produced occlude some small blood vessels, resulting in ischaemic damage (dead tissue) to body organs6. The damaged tissue releases more thromboplastins and a vicious cycle develops6.
Eventually all the clotting factors and platelets are consumed and bleeding results5. The patient is in the ironic situation of having both widespread blood clotting and a clotting deficiency. Bleeding occurs, petechiae develop in the skin and, if untreated, major haemorrhage can result7,8.
DIC can be subclinical, only detected on laboratory investigations, or may present with massive haemorrhage7,9 Bleeding is observed at vascular access points, GI tract, nose, genitourinary tract, intravenous cannulation sites and wounds8. Investigations2 reveal:
- Increase in prothrombin time
- Increase in partial thromboplastin time
- Increase in fibrin degradation products
- Decrease in platelets
- Decrease in fibrinogen
COMPLICATIONS
- Damaged kidneys – renal failure and anuria6
- Damaged liver – liver failure and jaundice6
- Damaged lungs – dyspnoea and cyanosis6
- Brain damage – convulsions or coma6
- Retinal damage – damaged sight or blindness6
- Pituitary damage – Sheehan’s syndrome6
- Major haemorrhage – hypovolaemia then death
NON-PREGNANCY TREATMENT AND CARE
- DIC is essentially a clinical diagnosis; laboratory tests confirm the diagnosis and guide replacement of blood component7
- Coagulation screen comprising:
- whole blood film
- FBC, especially platelet count8
- fibrinogen degradation products/D-dimers5,8,9
- prothrombin time (normal 10–14 seconds)8
- thrombin time (normal 1–15 seconds)8
- partial thromboplastin time (normal 35–45 seconds)
- fibrinogen levels (normal 2.5–4 g/l)8
- whole blood film
- Insert indwelling urinary catheter, ideally with a measuring chamber, and monitor urinary output
- Strict monitoring and recording of fluid balance
- Repeat the coagulation screen as clinically indicated
- Central venous pressure (CVP) monitoring10
- IVI fresh frozen plasma2, which contains all the clotting factors11
- IVI platelets6
- IVI packed cells (blood)6,11
- Identify and treat the underlying cause if possible2
- Institute high dependency care, transfer if necessary
- Respiratory support if indicated12
- Intravenous antibiotics for suspected septicaemia2
- Correct exacerbating factors, especially:
- dehydration
- acidosis
- renal failure
- hypoxia2
- dehydration
- Analgesia12
- Anticoagulation with heparin rarely used and requires supervision by a haematologist5,9
- Concentrates of blood factors (antithrombins and/or protein C) are effective in the non-pregnant state
- Recombinant activated protein C is used for sepsis in non-pregnant patients9
- Steroids may be used for precipitating factors
PRE-CONCEPTION ISSUES AND CARE
If a woman had DIC in a previous pregnancy, the recurrence risk will be that of the precipitating cause. Follow-up and de-briefing of any woman who had acute DIC is essential.
Women with chronic DIC may be at high risk of pregnancy complications and need to be assessed and advised by a haematologist before cessation of contraception.
- Miscarriage, particularly septic
- Septic, illegal termination4,14
- Hydatidiform mole10
- Placenta accreta and PPH10
- Retained dead fetus7,10
- Acute fatty liver of pregnancy10
- Placental abruption – most common cause
- Placenta praevia4,10
- Pre-eclampsia4,10
- HELLP syndrome4
- Amniotic fluid embolism7,10
- Mismatched blood transfusion7,10
- Breast/ovarian/uterine cancer4
- One of the above trigger factors
- Haemorrhage
- Ecchymoses (discoloured skin patches)
- Haematuria
- Shock
- Thrombotic complications in the brain, kidneys or lungs
- Acute pulmonary hypertension if the trigger was amniotic fluid embolism13
- Placental abruption = 1%10
- Infection/shock = 50–80%10
- All women with an associated condition (see opposite) should have a full blood count and coagulation screen13
- Before attributing a platelet count of ≤100 × 109/l to gestational thrombocytopenia, other causes of a reduced platelet count should be excluded13, especially DIC
- Multidisciplinary team approach, so call haematologist and anaesthetist
- Precipitating factor must be identified and treated immediately13
- Investigations – as for non-pregnancy (previous page)
- Blood samples for group and cross-match are especially important as operative delivery may be imminent
- Treatment – as for non-pregnancy (previous page)
- If haemorrhage commences, implement the institution’s major obstetric haemorrhage protocol
- Be aware that DIC can present chronically or acutely, the latter leading to major obstetric haemorrhage (a life-threatening event) and that the midwife might be the first to recognise the problem
- Remain with the mother, giving reassurance and oxygen, whilst preparing for an acute emergency
- Send for medical aid
- Initiate observations of vital signs, including measurement of blood loss and retaining blood-soaked items for inspection later
- Summon an assistant to prepare an IVI with blood giving set, and venepuncture equipment for the above blood samples
- Summon an assistant to attend the baby if the mother has delivered
- Insert indwelling urinary catheter and monitor fluid balance strictly
- Once medical aid arrives the midwife’s role is to assist the medical team and to care for the mother and baby
- If there is a fetus or placental tissue in utero, the mother is likely to be transferred to obstetric theatre, otherwise, the mother should be transferred to high-dependency care area, which may well be delivery suite
- Caesarean section may be required13
- Evacuation of the uterus if there are retained feto-placental products
- Close liaison with haematologist and blood bank
- Prepare for, and assist with, an emergency delivery
- Keep mother nil by mouth in case of general anaesthetic
- If a vaginal delivery – active management of the third stage and all perineal trauma must be sutured promptly
- Vigilant examination of the placenta and ascertain if it, or cord blood samples, need to be sent to the laboratory
- Accurate estimation of blood loss, observe for clotting and possibly retain for inspection by the medical team
- The coagulation imbalance usually resolves 24–48 hours post-delivery, and the low platelet count (thrombocytopenia) within a week11
- The baby may have been admitted to NNU
- Obstetric postnatal review – debrief; advice for future pregnancies
- Careful post-operative care paying particular attention to wound and cannulation sites for signs of bleeding
- Post-operative observations might be continued longer than usual
- Assist the mother to visit her baby on NNU
14.4 Von Willebrand’s Disease and Other Bleeding Disorders
EXPLANATION OF CONDITION
A familial haematological disorder, characterised by bleeding, was described in 1926 by von Willebrand in Finland3, hence the term von Willebrand’s disease (VWD). This is a condition in which there is either a defect, or deficiency, of the von Willebrand factor (VWF), a carrier protein for clotting factor VIII4. There are three5 basic types:
VWD is the most common inherited bleeding disorder in pregnancy6, and is inherited as an autosomal dominant condition. Hence, children of either gender may inherit the clotting deficiency7. However, women are more likely to present with symptomatic VWD due to menstruation and childbearing4. There are no ethnic differences8.
Non-pregnant patients usually present as young adults with excess bleeding, in the form of:
- Epistaxis (nosebleeds)1,5
- Menorrhagia (heavy and prolonged periods)1,2,5,9
- Bleeding after dental extraction or surgery1,5
- Bruising1,5
Screening tests8 entail:
- FBC – usually normal but a mild thrombocytopenia may occur in Type 2 patients8
- Serum ferritin
- Clotting screen – prothrombin time is normal, but the activated partial thromboplastin time may be prolonged
- Bleeding time – usually prolonged, but may be normal in mild forms of VWD8
- Platelet aggregation test – measures platelet efficiency8
- Von Willebrand factor antigen, factor VIII, Ristocetin cofactor (RiCof)
Other Bleeding Disorders
- Factor XI deficiency – similar problems to VWD Type 3
- Haemophilia A and B – female carriers can have low levels, and an associated bleeding risk
- Bleeding history and non-pregnant levels of factor VIII or IX should be identified
COMPLICATIONS
- Haemorrhage (severity varies from VWD Type 1 to 3)
- Joint bleeding and pain (in VWD Type 3)
- Anaemia and fatigue
- Pregnancy problems
NON-PREGNANCY TREATMENT AND CARE
Common Treatments
- Combined oral contraceptive pill (COCP) – to increase levels of factor VIII and von Willebrand factor, reduce menstrual blood volume, and prevent pregnancy8
- Iron supplementation – if clinical condition necessitates
- Vaccination against hepatitis A and B5
- Tranexamic acid (to inhibit bleeding) – slows the breakdown of blood clots; tablet form, or syrup for children1
- Desmopressin – a synthetic hormone (not a blood product) that enables VWF to be released into the blood circulation
- given iv at specialist centres1
- nasal spray is available
- given iv at specialist centres1
- Clotting factor concentrate – derived from human plasma, and used to treat severe cases of VWD1
Advice1
- Avoid aspirin
- Carry ‘green card’ from haemophilia centre at all times in case of accident or emergency
- For children, parents should inform the school
- Caution with certain holiday destinations, and a ‘travel pack’ of drugs may have to be issued
- Encourage exercise, but discourage contact sports
- Maintain a healthy lifestyle with an iron-rich diet
PRE-CONCEPTION ISSUES AND CARE
Women with a history of heavy menstrual bleeding since menarche, and a family history suggestive of a coagulation disorder, should be screened for coagulation disorders10.
Von Willebrand’s Disease
- There is no evidence that fertility is impaired4
- Pre-conception care aims to optimise maternal health
- The risk of a mother with Type 1 transmitting the condition to her child is 50% but only 33% of these will be clinically affected7
- Opportunity for pre-natal diagnosis for women with Type 3, whose genetic mutation is identifiable7
- If parents already have a child with Type 3, the chance of each subsequent child being affected is 25%7
Haemophilia
- Women with relevant family histories are assessed for carrier status and counselled over reproductive options7
Factor XI Deficiency
- Pre-natal diagnosis should be discussed if the woman’s condition is severe7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


