Clinical Manifestations (NEJM 2006;355:1244)
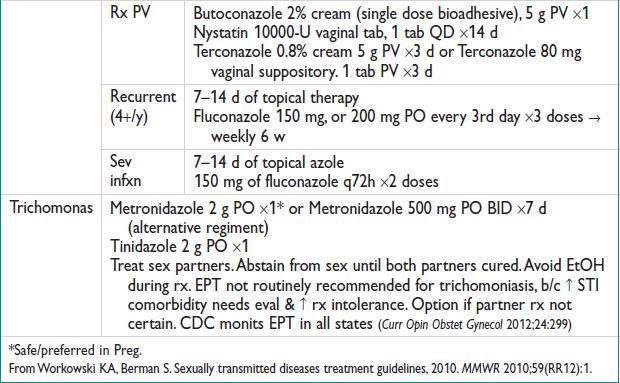
• BV: Copious, thin, whitish-gray, fishy-smelling discharge. Less likely pruritus.
• Candidiasis: Thick, white, curdy discharge. No odor. + Pruritus, dysuria, vaginal erythema.
• Trichomonas: Copious yellow to greenish, frothy discharge. Often foul odor. ± pruritus, postcoital bleeding, dysuria. ± vaginal or cervical erythema (“strawberry cervix”).
Diagnostic Studies (NEJM 2006;355:1244)
• BV: Nugent score = gold std, gram stain w/ scored bacteria & clue cells.
• Candidiasis: Presence of hyphae visible on KOH or wet mount. Yeast cx useful if pt c/o sx but negative wet mount, or if recurrent infxns.
• Trichomonas: Presence of mobile trichomonads on wet mount; ↑ PMNCs often present.
Treatment
BARTHOLIN GLAND CYST AND ABSCESS
Definition (J Obstet Gynaecol 2007;27:241)
• Bartholin gland secretes mucous vaginal lubrication. Located at ∼4- & 8-o’clock on labia minora bilaterally. Not palpable unless pathology. Usually women b/w 20–30 yo.
Etiology & Pathophysiology
• Blockage of gland outflow → accum of mucous → Bartholin duct cyst.
• Superficial infxn of a Bartholin cyst → Bartholin duct abscess. Polymicrobial. Most common bacteria are anaerobic & facultative aerobes.
• Bartholin cyst & abscess uncommon >40 yo. Consider biopsies of cyst wall to r/o cancer.
Clinical Manifestations and Physical Exam
• Small cysts are asx. Larger → vaginal pres or dyspareunia. Typically unilateral, round, & tense.
• Abscess = sev pain → difficulty walking, sitting, engaging in sex. May be tender w/ erythema/induration, purulent drainage.
• DDx: Epidermal inclusion cysts, mucous cyst of vestibule, cyst of canal of Nuck, Skene’s duct cyst (J Obstet Gynaecol 2007;27:241)
Treatment (See also Appendix of Common Procedures)
• Small, asx cyst requires no rx. OTC analgesics, warm compresses, & sitz baths may provide sx relief.
• Abscess may drain spontaneously. Immediate pain relief will occur w/ drainage.
• Surgical mgmt reserved for recurrences, abscesses, or large symptomatic cyst.
(1) I&D: Relief but incision can reseal → reaccumulation of fluid. Word catheter (or pediatric Foley) allows continued drainage & tract epithelialization. High recurrence rates after I&D. Leave catheter 4–6 w. May fall out before then.
(2) Marsupialization: Create new drainage site. Incise roof of cyst → sew edges of cyst wall to adj skin edge. Requires anesthesia, ↑ time, & placement of sutures. Low recurrence after marsupialization.
(3) Bartholin gland excision: Reserved for cyst that recurs repeatedly. ↑ risk of bleeding. Not performed if active infxn.
Antibiotic therapy often prescribed after surgical rx. Cx rarely change mgmt (Am Fam Physician 2003;68:135). Use broad spectrum abx, failure of clinical improv, consider MRSA.
UTERINE FIBROIDS
Definition
• Benign smooth muscle tumors, originating from myometrial tissue (leiomyoma).
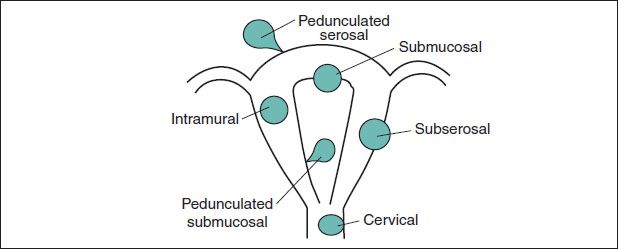
• Uterine fibroids can be classified based on their anatomical location.
Epidemiology (Obstet Gynecol Clin N Am 2011;38:703)
• By 50 yo, fibroids are found in ∼70% of whites & >80% of blacks. Indication for 30–40% of hysterectomies. Risks: >40 yo, black, FHx, nulliparity, obesity.
Pathology
• Gross: Pearly, round, well circumscribed. Size & location vary. Relatively avascular but surrounded by rich vasculature system → signif bleeding.
• Histology: Smooth muscle cells aggregated in bundles.
• Degenerating leiomyoma types: Hyaline (65%), myxomatous (15%), calcific (10%, mainly older women), cystic (4%, hylanized areas → liquefaction), fatty (rare), carneous (red) necrosis (esp pregnant pts, acute d/t outgrowing bld supply → acute musc infarction → sev pain & local peritoneal irritation).
• Leiomyomas do not transform into leiomyosarcoma. Likely represents a de novo neoplasm & is NOT a result of malig transformation of a benign tumor.
Figure 5.1 Fibroid location & nomenclature
Pathophysiology
• Fibroids are estrogen- (& progesterone-) sensitive tumors. Fibroids create ↑ estrogen environment → ↑ growth & size maint. ↑ estrogen conditions (obesity, early menarche) → ↑ fibroid risk.
Clinical Manifestations
• Mostly asx. Sx depend on size, location, & number. In general, the larger the fibroid, the larger the chance of sx.
• Vaginal bleeding = most common symptom; usually presents as menorrhagia.
• Other sx: Pelvic pain & pres, urinary frequency, incontinence, constip, infertility
• Evid sugg that myomas are the primary cause of infertility in a small # of women. Myomas that distort the uterine cavity & larger intramural myomas may have adverse effects on fertility (Fertil Steril 2008;90:S125).
Physical Exam & Diagnostic Studies
• Findings: Uterine enlargement, irreg uterine contour.
• Must r/o other causes of abn bleeding. Postmenopausal bleeding w/ fibroids should be evaluated the same way as women w/o fibroids.
• Imaging:
US: Defines pelvic anatomy & effective in locating fibroids.
SIS: Allows eval of uterine cavity, particularly if infertility or menorrhagia is a concern. Good for submucosal type.
MRI: Very accurate. Very expensive. Not practical depending on the clinical setting.
Hysteroscopy: Gold std for submucosal fibroid.
Treatment & Medications
• Observation: Asx fibroids do not require intervention, no matter their size.
• Medical mgmt (Obstet Gynecol Clin N Am 2011;38:703): Should be tailored to alleviating sx. Cost & s/e of rx may limit long-term use.
NSAIDs: No data to support use as sole agent for therapy. Good for dysmenorrhea based on role of PGs as pain mediators.
OC: 1st line. Combined OCs may control bleeding & pain, but progestin-only OCs w/ mixed results.
Levonorgestrel IUD: Beneficial for menorrhagia. ↑ rate expulsion & vaginal spotting.
GnRH agonist (Leuprolide 3.75–11.25 mg/m IM): Reversible amenorrhea in most, & 35–65% ↓ in size w/i 3 mo. Most useful in women w/ large fibroids. Induces menopause sx + ↓ bone density. Consider add-back therapy for prolonged use (>6 mo) or symptomatic pts. Use preop → ↓ uterine size before Surg.
Aromatase inhibs: Block ovarian & periph estrogen production → ↓ estradiol level after 1 d of rx. ↓ s/e compared to GnRH w/ rapid results. Little data.
Antiprogestins (Mifepristone 5 or 10 mg/d ¥ 6 mo): 26–74% ↓ in uterine vol & ↓ recurrent growth after cessation. S/e: Endometrial hyperplasia (dose-dependent) & transient ↑ in transaminase (monit LFTs).
• Nonsurgical mgmt:
UAE: IR injects PVA spheres into bilateral uterine artery → ↓ bld flow → ischemia & necrosis → ↓ size & sx. Postembolization syn may require hospitalization postop for pain control. Successful pregnancies occur after UAE, but long-term data limited.
US ablation under magnetic resonant guidance:
• Surgical Mgmt:
Hysteroscopic myomectomy: 1st line for symptomatic submucosal fibroids.
Myomectomy: Option for those desiring fertility or decline hysterectomy. Goal to remove visible & accessible fibroids, & reconstruct uterus. Via laparotomy or laparoscopy. Fibroids may recur. When myomectomy invades endometrial cavity (complete wall resxn) consider CS deliv @ 37–38 w gest (Obstet Gynecol 2011;118:323).
Hysterectomy: Definitive surgical rx. Satisfaction rate >90%.
ADENOMYOSIS
Definition & Pathogenesis
• Presence of endometrial glands & stroma w/i the uterine musculature
• Amt & degree of invasion vary. Diffuse or circumscribed focal glandular deposits.
Epidemiology
• Unclear etiology, but several theories. Possibly invagination of endometrium into myometrium, or misplaced stem cells or Müllerian remnants.
• 70–80% of cases seen in 4th & 5th decades. Only 5–25% of adenomyosis seen <39 yo.
• Estrogen & progesterone likely play role in dev & maint. Often develops during reproductive years & regresses after menopause. Risk factors: Parity, ↑ age
Clinical Manifestations & Physical Exam Findings
• Menorrhagia & dysmenorrhea. Many asx. Severity correlates w/ ↑ ectopic foci & extent of invasion. Less common complaints: Dyspareunia, CPP, infertility.
• Ectopic endometrial tissue → proliferates → enlarged globular uterus on exam
Diagnostic Workup (J Minim Invasive Gynecol 2011;18:428)
• Dx by histology. Uniform dx based on histology not yet developed.
• ↑ Ca-125 levels may be seen, but not proven to be helpful in mgmt or dx.
• TVUS preferred imaging technique = ill-defined myometrial heterogeneity, may be myometrial cysts (round anechoic areas). MRI may be complementary = large asym uterus, thickened junctional zone (innermost myometrial layer), no fibroids.
Treatment & Medications
• No medical therapy exists at this time to treat sx while allowing pts to conceive.
• Conservative, medical mgmt for symptomatic adenomyosis similar to 1° menorrhagia or dysmenorrhea. Goal = temporarily induce regression of adenomyosis.
• NSAIDs often given. May consider: Continuous oral contraceptives, progestins, Mirena IUD, danazol, & GnRH agonist.
• Surgical Mgmt (J Minim Invasive Gynecol 2011;18:428):
Hysterectomy = Std rx option for those done w/ childbearing.
Endometrial ablation = Treats menorrhagia sx. Less successful if ↑ penetration of adenomyosis into uterus is present.
UAE: Controversial. Less successful if fibroids also present.
Focal excision: Must be able to identify area, margins, & extent of dz. Low efficacy (50%). Addition of GnRH agonist ↓ relapse rates by 20% in 2 y. May have fertility & deliv implications depending on size & location of excision.
ENDOMETRIOSIS
Definition and Epidemiology (Obstet Gynecol 2011;118:69)
• Defined as presence of endometrial glands & stroma outside of nml location in uterus.
• Hormonally dependent → mostly reproductive aged women (6–10 prevalence %).
• Prevalence of 38% in infertile women & 71–87% w/ CPP.
• Risk factors: Early menarche (<11 yo), menstrual cycles <27 d, heavy & prolonged menses.
• Protective factors: ↑ parity, ↑ lactation periods, regular exercise (>4 h/w).
Etiology
• Most commonly accepted theory = retrograde menstruation → attachment of endometrial tissue on peritoneum. Other theories: Bld or lymph transport, stem cells from bone marrow, coelomic metaplasia.
Clinical Manifestations (Obstet Gynecol 2011;118:69)
• Often asymptomatic. Common: Dysmenorrhea, CPP, menorrhagia, dyspareunia.
• Pelvic pain described as pain before onset of menses (2° dysmenorrhea), deep dyspareunia (worse during menses), sacral backache during menses.
Diagnostic Workup/Studies (N Engl J Med 2010;362:2389)
• Physical exam findings: Uterosacral ligament nodularity, adnexal mass
• Laparoscopy w/ or w/o bx for histology (gold std). Path: Endometrial glands/stroma w/ varying amts of inflammation/fibrosis. Bld or hemosiderin-laden macrophages. Bx not req, but definitive.
• Visual appearance: Classical lesions = black powder burn. Nonclassical = red or white.
• No correlation b/w severity of visual dz & degree of pain or prog w/ rx.
• No serum markers or imaging studies useful in dx. Imaging studies (MRI, USG) only useful if + pelvic/adnexal mass (chocolate cyst).
• US: Ovarian endometriomas appear as cyst w/ low-level, homogenous internal echoes from old bld. TVUS = imaging of choice to detect deeply infiltrating endometriosis of rectum or rectovaginal septum. MRI rarely req.
Classification
• Numerous schemes proposed. ASRM classification most common. Value = uniform recording of OR findings & comparing therapeutic interventions.
• ASRM criteria: Stage I (minimal) → Stage IV (sev). Based on extent & location of endometriosis lesions seen during operative procedure.
Treatment & Medications
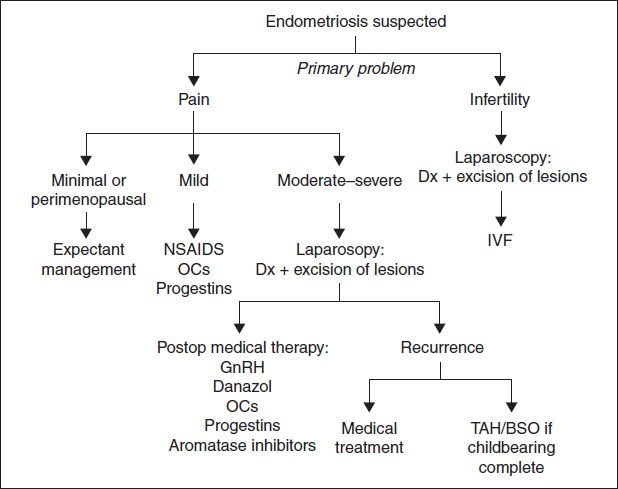
• Best treated medically w/ surgical backup. Surgical mgmt reserved for large endometriomas, palpable dz, or infertility (Fertil Steril 2008;90:S260).
Figure 5.2 Management algorithm for endometriosis
• Medical therapy (Fertil Steril 2008;90:S260): Medical suppressive therapies are ineffective for infertility (Int J Gynaecol Obstet 2001;72:263)
NSAIDs: COX inhibs → ↓ PG synthesis → ↓ pain & inflammation
OCs: Can be used in cyclic or continuous fashion. Amenorrhea often result of continual therapy, which is often beneficial for pt w/ pain sx.
Progestins: Antagonize estrogenic effects on endometrium → decidualization → eventual endometrial atrophy.
Medroxyprogesterone acetate 20–100 mg PO QD or 150 mg IM q3mo (depot)
NETA 5 mg QD, ↑ 2.5 mg QD until amenorrhea or → 20 mg/d max reached
Mirena IUD. Unk MOA. Is efficacious, but not approved by FDA for this use.
GnRH agonists: ↓ signaling of HPA-axis → ↓ estrogen → amenorrhea & endometrial atrophy. Nasal spray (nafarelin acetate) or depot formulation (leuprolide acetate) q1–3mo. S/e = menopause sx + ↓ bone density. Add-back therapy w/ progesterone or combo (estrogen/progesterone) used to ↓ s/e. Theory = amt necessary to prevent menopause sx < amt to stimulate endometriosis. Can be started immediately w/ GnRH agonist administration. Does not diminish efficacy of pain relief. Norethindrone acetate (only hormone FDA approved for add-back therapy) 5 mg PO QD w/ or w/o CEE (premarin) 0.625 mg QD × 12 mo.
Danazol (600–800 mg QD): Inhibit LH surge → chronic anovulatory state. Substantial androgenic & hypoestrogenic s/e that limit clinical utility.
Aromatase inhibs: Still investigational. Not definitive therapy.
• Surgical therapy (Fertil Steril 2008;90:S260): Relief of pain after surgical rx = 50–95%. Laparoscopic rx of visible endometriosis improves pain. All visible lesions should be treated.
Conservative Surg (diagnostic laparoscopy, lysis of adhesions, ablation/fulguration of visible implants, normalization of anatomy) = 1° approach for symptomatic or large endometriomas b/c medical therapy will not lead to complete resolution. Cyst excision in endometriomas has improved outcomes over simple cyst drainage.
LUNA: Disrupts efferent nerve fibers in the uterosacral ligaments → ↓ uterine pain for intractable dysmenorrhea. No benefit > conservative Surg alone.
Presacral neurectomy: Interrupts symp innervation to uterus @ level of superior hypogastric plexus. Benefit in midline pain only. Technically challenging w/ signif risk of bleeding. S/e: Constip, urinary dysfxn.
Hysterectomy (TAH/BSO): For those w/ debilitating sx, have completed childbearing, & failed other therapies. Long-term adherence w/ HRT req to prevent ↑ risk of mortality a/w BSO prior to menopause (Obstet Gynecol 2010;116:733). Use estrogen/progesterone therapy d/t risk of unopposed estrogen more likely to cause growth of endometrial implants.
• Surg, followed by medical therapy offers longer sx relief than w/ Surg alone. OC, progestins, GnRH analogs, & danazol have been shown to ↓ pain & ↑ time until recurrence (Fertil Steril 2008;90:S260; Hum Reprod 2011;26:3).
RECURRENT ABNORMAL UTERINE BLEEDING (AUB)
Definition and Etiology
AUB: Menstrual flow outside of nml vol, duration, regularity, or frequency. Excessive bld loss is based on pts’ perception.
Pathophysiology
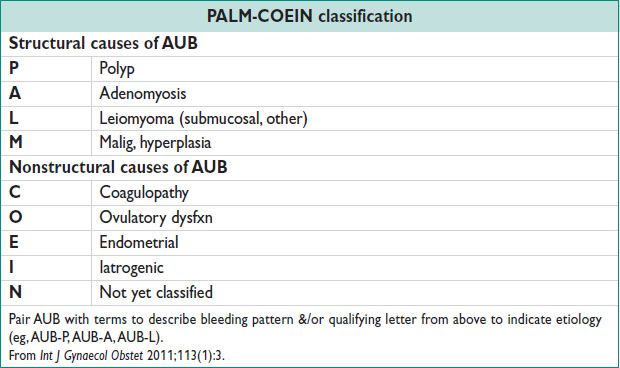
• See PALM-COEIN table.
• Anovulation → no cyclic progesterone production → ↑ estrogen → ↑ endometrial proliferation → amenorrhea → eventually, endometrium overgrown & structurally fragile → random & dyssynchronous endometrial sloughing → irreg vaginal bleeding → AUB/menorrhagia. An anovulatory pt is always in follicular phase of ovarian cycle & in proliferative phase of endometrial cycle. No luteal or secretory phase b/c no cycles. Unopposed estrogen ↑ risk of endometrial hyperplasia.
Differential Diagnosis
• Always consider Preg or related complications (SAB, ectopic).
• Teens: MCC d/t persistent anovulation d/t immaturity or dysregulation of HPA (= nml physiology), coagulopathy, contraception, infxn, tumor.
• Reproductive age (19–39 y): Structural abnormalities (PALM), anovulatory cycles, contraception, endometrial hyperplasia. Cancer less common but may occur.
• Perimenopause: Endometrial hyperplasia, cancer, anovulatory bleeding d/t declining ovarian fxn (= nml physiology).
Diagnostic Workup (BMJ 2007;334:1110; Obstet Gynecol Clin N Am 2008;35:219)
• Detailed history & physical exam, including bimanual exam to evaluate uterus & speculum exam to evaluate cervix & vagina. Complete menstrual Hx is essent & can provide dx w/ suff confidence that rx can begin empirically.
• Regular, heavy menses usually anatomical lesion or bleeding d/o.
• Lab tests: Preg test, CBC, TSH. Consider pap smear & chlamydia testing. R/o bleeding disorders, particularly in teens. Serum progesterone in luteal phase >3 ng/mL sugg recent ovulation, but timing of test difficult w/ irreg menses.
• An EMB is not always req, except for >45 yo. Consider before rx if long-term unopposed estrogen exposure present, regardless of age.
• Imaging reserved to evaluate finding on physical, when sx persist despite rx, or suspicious for intrauterine pathology (AUB-P or AUB-L).
Treatment & Medications (Obstet Gynecol Clin N Am 2008;35:219; Menopause 2011;18:453)
• Treat underlying etiology. If no ↑ risk of endometrial hyperplasia, cancer, or underlying structural abnormalities, start empiric medical rx. Expect improv in 3 mo. Failure to improve → need to r/o other etiologies before changing mgmt. See also Chap. 2 for acute bleeding.
• Rx goals: (1) reverse abnormalities of endometrium d/t chronic anovulation, (2) induce or restore cyclic predictable menses of nml vol & duration.
• Surgical mgmt:
Acute surgical mgmt: Rare. If hemodynamic unstable, bleeding refrac to 2 doses of IV premarin, or bld loss that cannot be replaced w/ xfusion, OR mgmt (D&C) req. Should continue medical therapy after D&C. Informed consent should include hypogastric artery ligation & hysterectomy should D&C fail. Uterine artery embolization may be considered as an alternative, if available.
Endometrial ablation: High success rate. 25–50% are amenorrheic, & 80–90% have ↓ bleeding. Effective alternative to hysterectomy. ↑ success if pretreated w/ progest or GnRH. R/o cancer prior to Surg. Up to 1/3 will eventually elect for hysterectomy.
Hysterectomy: High satisfaction, but more morbidity & poor choice in pts w/ medical conditions w/ high risk for Surg.
POSTMENOPAUSAL BLEEDING
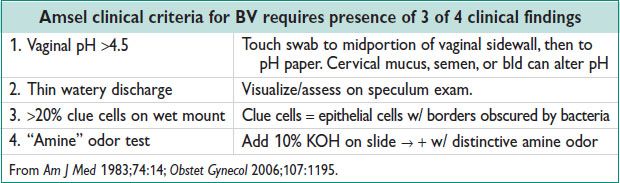
Definition, Epidemiology, & Etiology (Obstet Gynecol 2010;116:168)
• PMB: Vaginal bleeding occurring after ≥12 mo of amenorrhea
• PMB “is endometrial cancer until proven otherwise.” Malig w/ PMB = 1–14%. Predictive value depends on age & risks: Obesity, HTN, diabetes, low parity.
• Caused by cancer (10%), atrophy (60–80%), endometrial hyperplasia (2–12%), HRT (15–25%). Tamoxifen increases endometrial cancer risk. TVUS less useful d/t subepithelial stromal hypertrophy. Therefore any bleeding w/ tamoxifen → w/u.
Diagnostic Workup (Obstet Gynecol 2010;116:168)
• Comprehensive H&P: Pelvic exam to evaluate rectal, vulvar, vaginal, or cervical origin.
• Goal of endometrial eval: (1) exclude malig, (2) rx based on proper etiology (anatomic vs. nonatomic pathology)
• Endometrial eval:
Transvaginal US allows initial screening in some protocols. An EMS on TVUS <5 mm, has a risk of malig of 1:917. PPV 9% & NPV 99%. Sens 90%, spec 48% for endometrial cancer. About 50% of pts w/ initial TVUS → further eval (Obstet Gynecol 2009;113:462). Limitations: EMS not always visible, particularly w/ prior Surg, fibroids, obesity, adenomyosis. Incidental thick EMS in an asx pt does NOT require intervention. Often d/t polyps (82%) → no intervention b/c negligible risk that an asx polyp (ie, no bleeding) will harbor cancer (1:1000).
EMB: Accurate for excluding cancer, but only samples small focus of endometrium. Sens 99%, spec 98%. False negative ∼10%. High rate of insuff or failed sampling (0–54%) → further eval (Maturitas 2011;68:155).
Sonohysterography: Imaging w/ saline infusion (SIS) overcomes some TVUS limitations.
3D US & Doppler adds no additional information at this time.
D&C: Useful when unable to obtain EMB (cervical stenosis, pt intolerance, etc.). Invasive: 1–2% complication rate. May miss 10% of endometrial lesions, & of these up to 80% are polyps.
Figure 5.3 Management of postmenopausal bleeding