Fig. 2.1
Basic organizational structure of the follicular associated epithelium (FAE). The main functional component of the FAE is the M (microfold) cell, which is specialized in transepithelial antigen transport. Transported antigen is taken up and processed by dendritic cells (DCs) and antigen-presenting cells (APC) which present it to naïve T cells
Evidence for Barrier Dysfunction in the Pathogenesis of IBD
Several lines of investigation indicate that disruption of the epithelial barrier may either instigate or perpetuate chronic intestinal inflammation. Abnormal intestinal permeability has been established among patients with Crohn disease and their healthy first-degree relatives, and may represent a primary abnormality predisposing to excessive antigen uptake, continuous immune stimulation, and eventually mucosal inflammation. Additionally, epithelial cell death, particularly Paneth cell drop out has recently been demonstrated to induce terminal ileal inflammation in mice and associated with Crohn’s disease in humans [9]. Interestingly, barrier dysfunction was observed directly in a cohort of patients with Crohn’s disease by confocal endomicroscopy, and this dysfunction was predictive of relapse [10]. Potential etiologic factors for barrier dysfunction in Crohn disease may be environmental or genetic. Smoking and nonsteroidal anti-inflammatory drugs both potentially affect gut permeability and are variably associated with IBD [11]. Furthermore, polymorphic variants in several IBD-associated genes (OCTN, DLG5, JAK2, MYO9B) appear to primarily affect epithelial permeability and may lead to inappropriate exposure of the mucosal immune system to luminal antigen [12–15].
Innate Immune Cells
The immune system can be divided into two main branches: innate and adaptive immunity, characterized by differences in regard to specificity and timing of response. In contrast to adaptive immune cells, typically represented by T cells, which require some time for priming and clonal expansion for full effectiveness, cells of the innate immune system rapidly seize upon potential threats to the host. The rapidity of response occurs because the receptors used to quickly detect invading microorganisms or toxic macromolecules are germ-line encoded, invariable, and predetermined to recognize a repertoire of bacterially/virally associated carbohydrate and lipid structures. These pathogen recognition receptors may be membrane-bound (i.e., Toll-Like Receptors—TLRs) or cytoplasmic (i.e., Nucleotide-binding Oligomerization Domain family members—NODs). Innate immune cells primarily responsible for immediate bactericidal activity include professional phagocytic cells (macrophages and neutrophils), eosinophils, and lymphoid cells that have evolved to fulfill innate rather than adaptive immune functions. As a prelude to the discussion of the relationship between luminal bacteria and lymphocytes, background information regarding the macrophage, dendritic cell, and the atypical lymphocytes (NKT and γδ T cell) will be presented.
Macrophages
Macrophages are phagocytic cells present in large numbers throughout the gastrointestinal mucosa. In fact, the GALT contains the largest reservoir of macrophages within the body [16]. Macrophages mature continuously from peripheral blood monocytes that are recruited to sites of mucosal inflammation [17], while neutrophils are produced and lost in large numbers each day. In the noninflamed intestine macrophages differ from peripheral monocytes in their hyporesponsiveness to TLR ligands, diminished ability to prime adaptive immune responses, yet preserved capacity for phagocytosis and intracellular killing [17]. However, in the setting of pathogen invasion and inflammation, intestinal macrophages freshly recruited from blood monocytes, instead of developing a primarily scavenger function, rapidly display a pro-inflammatory phenotype marked by abundant cytokine production and accessory cell function. The conversion to this pro-inflammatory phenotype is stimulated by ligation of their pathogen recognition receptors.
Activated macrophages express membrane-bound receptors specific for opsonized particles and pathogens, complement, and common bacterial proteins (i.e., mannose receptor, TLR, NOD). The complement receptor for C5a is largely responsible for the movement of macrophages to sites of inflammation, and reduced expression as seen in patients with acquired immunodeficiency syndrome results in susceptibility to mycobacterial infection [18]. Recognition of pathogens through these receptors leads to phagocytosis and intracellular degradation. Activation of macrophages also results in the secretion of bioactive molecules called cytokines. Cytokines are proteins produced and secreted by one cell that affect the function of another cell, thus allowing cell–cell communication in the absence of cell contact. The cytokine TGF-β produced by activated macrophages is also a potent chemoattractant leading to the further recruitment of macrophages and neutrophils to the area of inflammation [19]. Not only is cytokine secretion important for the augmentation of phagocytic intracellular killing, but it also represents a critical link between innate and adaptive immunity, a topic discussed more fully later. Dendritic cells are also phagocytic cells, but their most important role is that of antigen presentation and activation of T cells.
Dendritic Cells
The dendritic cell (DC) is the most potent inducer of adaptive immune responses. Current evidence supports the model that intestinal DCs continuously migrate in an immature or tolerogenic form scavenging apoptotic cells and acquiring antigen sampled either directly from the lumen [20] or shuttled from the lumen through M cells (Fig. 2.1) [21]. T cells activated by Peyer’s patch-derived DCs (intestinal DCs) produce IL-4, IL-10, and TGF-β, three cytokines that promote the induction of T cells with regulatory function (discussed more fully later). This cytokine profile indicates that intestinal DCs may have a role in the induction of regulatory T cells and/or B cell responses. Upon activation through microbial products or pro-inflammatory molecules, mature DCs migrate to T cell areas of the GALT where they induce effector rather than tolerogenic T cell responses. Intestinal DCs can induce the mucosal homing receptor α4β7 and the chemokine receptor CCR9 on T cells, suggesting that they can instruct a T cell to home back to the original site of activation [22, 23].
Upon acquisition of antigen, DCs process the antigen within the cell and load antigenic peptide onto MHC class II molecules displayed on the surface membrane. DCs fully loaded with antigen migrate to the draining mesenteric lymph nodes where the greatest opportunity exists for presentation of a particular MHC–peptide complex to T cells bearing the T cell receptor (TCR) specific for the antigen being presented. Under normal circumstances, these antigen-loaded DCs express low levels of co-stimulatory molecules and cytokines, stimulating preferentially the differentiation of regulatory T cells. As opposed to these tolerogenic DCs isolated from noninflamed bowel, DCs stimulated with pathogenic bacteria or isolated from sites of inflammation are strongly immunogenic, expressing on the cell surface high levels of co-stimulatory molecules, adhesion molecules, and abundantly produce cytokines. The resultant T cell activation will be discussed further below.
Atypical Lymphocytes
The atypical lymphocytes NKT (Natural Killer T) cells and γδ T cells also play a role in the innate immune response. NKT cells mature in the thymus and recognize lipid antigen (presumably bacterial) presented on the “MHC-like” complex Cd1d. NKT cells secrete large amounts of pro-inflammatory cytokines and readily kill infected cells or tumor cells. The potential importance of these cells to the pathogenesis of ulcerative colitis has been recently described. Pro-inflammatory cytokine-producing NKT cells isolated from patients with ulcerative colitis have cytotoxic activity against colonic epithelial cells in culture and produce large amounts of IL-13 [24].
T cells that express gamma and delta TCR chains (γδT cells), rather than the typical αβ TCR chains, are atypical lymphocytes that only exist in significant quantity in epithelial tissues. In the intraepithelial space, γδT cells make up approximately 10 % of the total number of T cells, the remaining majority being CD8+ T cells [25]. Unlike conventional T cells, γδT cells do not require the thymus for development [26], do not recognize antigen in association with MHC class I or II [27], and do not participate in adaptive immune responses [28]. While their precise function in humans remains enigmatic, experimental evidence suggests that γδ T cells perform different functions depending upon tissue distribution and the local microenvironment [29].
Development of the Epithelial Barrier and Innate Immune Cells
While the functional properties of the fetal intestinal epithelial barrier are unknown, progressive maturation of epithelial tight junctions has been identified histologically [30]. It is apparent that neonatal intestinal permeability is higher than that in adulthood; [31] yet, its relationship to the development of an efficient mucosal immune system by facilitating exchange of dietary antigen, enteric colonizing bacteria, and maternal antibody or trophic factors is unknown. Interspersed within the intestinal epithelium, M cells sample the lumen and deliver antigen to DCs positioned below the FAE. M cells are present by week 17 of gestation following the appearance of lymphoid aggregates. Indeed, it appears that lamina propria lymphocytes and luminal bacteria promote M cell differentiation from enterocytes [32]. A second form of differentiated enterocyte is the Paneth cell, a specialized epithelial cell present within small intestinal crypts and dedicated to the production of various antibacterial peptides, like defensins. The Paneth cell is also present by 17 weeks of gestation [33]. Macrophages and DCs appear in the fetal intestine by 12 weeks, but little is known about their functional maturational state; however, TGFβ isoforms have recently been critically associated with maintenance of a toleragenic state [34, 35].
Data available on neonatal macrophages document defective production of pro-inflammatory cytokines such as IL-12 and TNFα [36].
Evidence for Innate Immune Cellular Dysfunction in the Pathogenesis of IBD
At least three pieces of evidence exist indicating a major role for innate immune cells in the perpetuation of human chronic intestinal inflammation. First, the strongest genetic association to date with small bowel Crohn disease is the loss-of-function polymorphisms in the bacterial sensing gene CARD15/NOD2. Whether the ultimate pathogenic dysfunction rests in Paneth cells [37] or monocytes [38], it appears certain that dysregulation in the detection of and/or responsiveness to enteric bacteria by innate immune cells promotes chronic inflammation in this subgroup of patients. The second piece of evidence rests in the efficacy of immune therapy targeted against pro-inflammatory cytokines produced by innate immune cells. Activated macrophages produce large amounts of TNF-α and IL-12, two cytokines responsible for the recruitment and activation of pathogenic effector T cells. The efficacy of anti-TNF-α therapy is established [39], and promising anti-IL-12 studies are currently ongoing [40]. The third piece of evidence is the presence of a defective acute inflammatory response to injury and bacterial products in Crohn disease patients, demonstrated by reduced neutrophilic infiltration, IL-8 production and vascular flow [41], and corroborated by the clinical improvement provided by the administration of GM-CSF, supposedly by improving macrophage and neutrophilic function [42]. This failure of clearance of bacteria and inflammatory debris indicates a disruption in the autophagy pathway. Autophagy, (process of cell self-digestion), has received significant investigative interest recently as many of the 90 Crohn’s associated genetic loci fall generally within the category of autophagy homeostatic function (i.e., ATG16L1 and IRGM) [43]. This avenue of research holds great promise in the near future.
Adaptive Intestinal Immunity and IBD
T Cells
Within the lamina propria of the intestine CD4+ and CD8+ T cells bearing the conventional αβ TCR are roughly equally represented. The intraepithelial space contains unusual and enigmatic T cell populations bearing the γδ TCR and CD8+ cells with αα homodimeric expression of TCR (discussed briefly earlier). As current understanding of both human and experimental IBD emphasizes the primary importance of activated CD4+ TCR αβ T cells to disease pathogenesis, the discussion later will focus on activation of CD4+ T cells and the homing pattern that ensues. Upon maturation in the thymus, naïve T cells (cells which have yet to experience antigen exposure) circulate the lymphatic tissues in search of its cognate MHC–peptide complex. Constitutive expression of the selectin CD62L (l-selectin) and the chemokine receptor 7 (CCR7) ensures that naïve T cells bind to glycosylated CD34 and the chemokine ligand 21 expressed on endothelial cells of high endothelial venules [44]. The interaction of CD62L and CD34 (among other glycosylated endothelial molecules) promotes a rolling action of the T cell across the endothelial surface. The T cell surface adhesion molecule LFA-1 binds to ICAM-1 and ICAM-2 promoting firm adhesion and crossing of the endothelial lining into the lymphoid tissue. Within the underlying lymphatics await DCs loaded with antigen in variable states of activation. Naïve T cells search the DC selection for a recognizable MHC–peptide complex. If none is found the cells exit the lymph node, return to the circulation, and reenter other nodes to repeat this process.
Eventually, a naïve T cell finds its specific antigenic mate and receives the appropriate activation signals upon ligation of its TCR (Fig. 2.2). A full T cell activation requires two signals: signal 1 is delivered by TCR stimulation and signal 2 is a co-stimulatory signal provided by secondary accessory pathways. Among the latter, co-stimulation through CD28 by B7.1 or B7.2 results in T cell activation; in contrast, co-stimulation through CTLA-4 by B7.1 or B7.2 results in T cell inhibition. In addition, TCR signaling in the absence of co-stimulation results in anergy, defined as a lack of response upon re-exposure to the same antigen in the future. A key consequence resulting from activation of CD4+ T cells is cytokine secretion, and the particular pattern of cytokines secreted orchestrates the type of the ensuing immune response (i.e., directed against intracellular pathogens, parasites, etc.). The phenotype of the mature effector T cell depends upon in large part the cytokine milieu available to the T cell while undergoing the activation and differentiation program. While insight into these complex events is in constant evolution, current understanding is as follows (Fig. 2.3). Activation of CD4+ T cells in the presence of IL-12 and absence of IL-4 results in a T helper 1 (Th1) phenotype, resulting in IFNγ-producing cells effective in the control of intracellular pathogens. Cells activated in the presence of IL-4 acquire a T helper 2 (Th2) phenotype, generating IL4-, IL5-, and IL13-producing cells effective in allergic responses and clearance of parasitic infections. Finally, activation in the presence of IL6, TGFβ and IL-23 results in cells expressing the recently described Th17 phenotype, i.e., IL17 and IL6-producing T cells responsible for acute inflammation and recruitment of granulocytes [45, 46]. Full activation of T cells takes 4–5 days and is accompanied by clonal expansion and remarkable changes in the homing behavior of these cells.
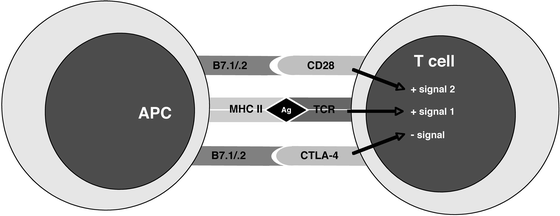
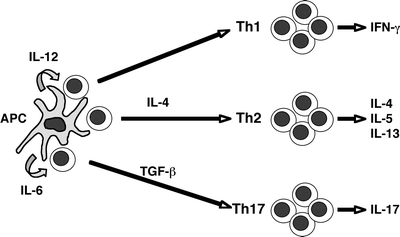

Fig. 2.2
Major molecular receptor-ligand pairs and co-stimulatory/inhibitory signals involved in antigen-specific stimulation of a T cell. APC antigen-presenting cell; MHC II major histocompatibility complex class II; Ag antigen; TCR T cell receptor; CTLA-4 cytotoxic T lymphocyte antigen-4

Fig. 2.3
Current understanding of the pathways mediating differentiation of naïve T cells into the three major types of effector Th cells (Th1, Th2, and Th17). The ultimate functional phenotype of mature effector T cells depends upon the type and amount of antigen, and the cytokine milieu present at the time of activation. APC, antigen-presenting cell
Once activated, these effector T cells leave the lymph node returning to the circulation, thus allowing a locally generated immune response to promote defense at distance throughout the gastrointestinal tract [47]. Activated T cells express unique adhesion molecules that direct the cells to sites of inflammation, and indeed T cells primed by mucosal DCs are destined to return to the gut through the upregulation of two surface molecules, α4β7 integrin and the chemokine receptor CCR9 [22]. The integrin α4 binds to the vascular adhesion molecule VCAM-1, a molecule expressed on vascular endothelium only at sites of inflammation. The α4β7 integrin recognizes the more ubiquitous vascular endothelial molecule MAdCAM-1, directing migration to the intestinal lamina propria [48, 49], while αEβ7 is uniquely expressed on T cells destined to the intestinal intraepithelial space. MAdCAM-1 has been shown to be upregulated during exacerbations of IBD, and thus represents a promising target for therapeutic intervention [49].
Effector T cells appear to enter nearly all tissues in limited numbers. Recognition of cognate antigen within the tissue results in T cell cytokine production. Pro-inflammatory cytokines, such as TNF-α, stimulate endothelial cells to upregulate adhesion molecules such as E-selectin (that recruits monocytes and neutrophils), and VCAM-1 and ICAM-1 (both of which recruit activated T cells). TNF-α and IFN-γ likewise act to alter the vascular permeability, endothelial cell shape, and blood flow, resulting in enhanced infiltration of inflammatory cells into the tissue. These inflammatory cascades set in motion by activated T cells and the significant structural alterations they cause in the inflamed tissue eventually trigger the signs and symptoms characteristic of active IBD.
Thus, naïve T cells circulate nearly all tissues due to the expression of CD62L, CCR7, and LFA-1. Activation of T cells in the GALT leads to expression of α4β7 and CCR9 expression, two molecules that produce selective homing to the intestine through endothelial expression of MAdCAM-1 and CCL-25. Mucosal inflammation results in upregulation of MAdCAM-1, in addition to other endothelial ligands, and enhanced recruitment of pro-inflammatory T cells to the inflamed organ.
Regulatory Cells (TREG)
It is now well established that subpopulations of CD4+ T cells with regulatory potential exist in both mice and humans. Current models describe three major and not necessarily mutually exclusive subtypes of Tregs: the “naturally occurring” CD4+ CD25+ cell, the IL-10- and TGF-β-producing Tr1 cell, and the TGF-β-producing Th3 cell derived through oral tolerance [50]. While accurate description of this rapidly evolving field is a moving target, the more studied and better understood Treg cell type is the CD4+ CD25+ T cell expressing the transcription factor FOXP3. The importance of this cell type to murine colitis and its capacity to suppress mucosal inflammation are well established [51, 52]. However, although this type of Treg has also been described in IBD [53], the function of these cells in the periphery and the gut of patients with IBD has yet to contribute to a better understanding of IBD pathogenesis.
B Cells
B cells of the GALT work in close collaboration with the epithelium to export secretory IgA (sIgA) and to some extent secretory IgM (sIgM) to enhance mucosal defense from intestinal pathogens. Mucosal B cells exhibit a predominant IgA class switch and, upon differentiation to mature plasma cells, produce approximately 3 g of sIgA per day [54]. Over 80 % of all human plasma cells are found in the gut, and nearly all these plasma cells produce IgA [55]. Mucosal plasma cells produce primarily dimeric or polymeric forms of IgA. The joining or “J” chain of polymeric IgA spontaneously interacts with the polymeric Ig receptor expressed on the basolateral surface of epithelial cells facilitating exportation of the sIgA to the gastrointestinal lumen [56]. Once in the lumen, sIgA coats commensal and potentially pathogenic bacteria. This coating may promote M cell-mediated bacterial uptake and target presentation to intestinal DCs and macrophages, providing a barrier to bacterial penetration as well as a positive feedback loop to enhance secretory immunity [57].
Development of Adaptive Immune Cells
Lymphocytes begin to populate the lamina propria by 12 weeks of gestation (after the thymus matures) and are primarily CD4+ cells [58]. Organized lymphoid tissue of the small bowel (Peyer’s patches) consisting of B cell follicles, and interfollicular CD4+ and CD8+ T cells are detected by 16–18 weeks of gestation [58]. Dendritic cells are detected at 19 weeks, representing the last of the required cellular components of an adaptive immune response [59]. Neither the T cells nor the B cells of the Peyer’s patch exist in an activated state within the fetal intestine. Indeed, the number of Peyer’s patches increases from 80 to 120 at birth to 250 by adolescence [60]. Without antigenic stimulation, there are no IgA secreting cells; yet, within 2 weeks of birth IgA and IgM secreting plasma cells are present within the lamina propria [61].
Evidence for Adaptive Immune Cellular Dysfunction in the Pathogenesis of IBD
There are multiple lines of evidence strongly supporting the notion that activated CD4+ T cells are a central feature of human IBD. First, T cell-driven animal models of colitis mimic human IBD in both histologic features and susceptibility to similar treatment regimens [24, 62–64]. In human IBD, IL17-secreting Th17 cells have now been an established observation in Crohn’s disease, while ulcerative colitis represents an atypical Th2 response [24, 65, 66].
Furthermore, the IL23 receptor variant associated with Crohn’s disease results in impaired IL17 production and is a protective genetic variant [67].
As mentioned previously, established and emerging therapy for human IBD is directed towards the destruction or deterrence of activated effector T cells, or blockade of Th1-driving cytokines [68]. Clonal populations of T cells have been described in patients with Crohn disease [69]. A similar development of T cell clones has been described in an animal model of IBD. Importantly, these T cell clones specifically recognize endogenous gut bacteria and are proven pathogenic in their ability to mediate colitis when transferred into noncolitic recipient mice [70]. Finally, the presence of antibodies unique to patients with IBD directed against microbial antigen signifies a potentially pathogenic adaptive immune responses against the intestinal flora [71].
Putting it all Together: Integrating Gut Microbes, Epithelial Cells, and Lymphocytes in the Pathogenesis of IBD
With rare exception, murine models of IBD require the presence of intestinal bacteria for the development of colitis. The presence of antibodies to bacterial antigen in most patients with IBD and the beneficial effects of antibiotics and probiotics suggest an important role of bacteria in the instigation or perpetuation of human IBD. Thus, understanding the interaction between gut flora, epithelial cells, and adaptive immune cells is important.
Intestinal epithelial cells encounter commensal and pathogenic bacteria routinely, and constitutively express membrane bound and intracellular receptors to sense gut microbes. The membrane bound TLR2 and 4 have been described in mouse and human intestinal epithelial cells lines [72]. Experimental evidence suggests that interaction between commensal gut flora and TLRs protects against colitis and enhances the mucosal barrier [73, 74]. An additional set of intracellular bacterial sensing receptors, NOD1 and NOD2, exists to detect intracellular pathogens. Experimental evidence indicates an antibacterial effect of the NOD2 protein in infected intestinal epithelial cell lines [75]. The importance of NOD2 in epithelial cell defense systems (particularly Paneth cells of the terminal ileum) underscores the relevance of polymorphisms in the NOD2/CARD15 gene in the pathogenesis of Crohn disease [76, 77]. It is established that the downstream signaling pathways of TLRs and NODs lead to activation of NF-κB responsive genes mediating inflammatory responses, but the precise details of these important cascades are beyond the scope of this chapter. Thus, intestinal epithelial cells directly sense enteric bacteria, and this interaction is essential to normal barrier function.
Potential pathogenic and probably commensal bacteria may contact immune cells of the GALT in at least three ways (Fig. 2.4). First, infected epithelial cells may undergo apoptosis (either spontaneously or killed by effector immune cells), and apoptotic fragments containing bacteria may be ingested by resident phagocytic cells (macrophages and DCs) and subsequently presented to host T cells. In this fashion, bacterial antigen may be presented to CD4+ T cells through MHC class II-TCR interaction and/or CD8+ cytotoxic T cells, as antigen from apoptotic fragments may be presented as “self” on MHC class I molecules [78]. Second, DCs and macrophages may acquire bacteria directly from the environment. DCs have been shown to directly sample intestinal antigen from the lumen, and both cell types engulf and kill whole bacteria, presenting bacterial antigen on MHC class II to CD4+ T cells. Third, lymphocytes may contact bacterial antigen directly in the absence of antigen-presenting cells. T lymphocytes have been shown to express TLRs and in certain model systems, respond by proliferation and cytokine secretion [79]. Thus, multiple pathways exist with the potential of presenting enteric bacterial products to CD4+ T lymphocytes.
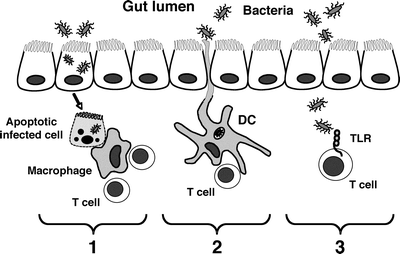

Fig. 2.4
Potential pathways through which bacterial antigens can activate mucosal T cells. (1) Apoptosis of infected epithelial cells; (2) luminal sampling and acquisition of bacteria by mucosal dendritic cells (DC); (3) direct contact of translocated bacteria with T cells mediated by Toll-like receptor (TLR) recognition
As previously pointed out, the T cell response to recognition of bacterial antigen on MHC class II molecules through the TCR depends upon the expression of co-stimulatory molecules on the antigen-presenting cell (Fig. 2.2). Activation of the CD4+ T cell results in cytokine secretion and the pattern of cytokines secreted determines the type of the ensuing immune response (i.e., directed against intracellular pathogens, parasites, etc.). These pro-inflammatory cytokines incite and amplify a vigorous immune response through many factors including increased blood flow, increased vascular permeability, and recruitment of effector immune cells. Activated CD4+ T cells also stimulate B cells through expression of the cell surface molecule CD40 ligand. CD40–CD40 ligand interaction results in B cell proliferation, variation in the class of antibody secretion (i.e., IgG, IgA, etc.), and initiation of antibody secretion. Following this multifactorial antigen-driven immune response, a series of secondary events also unfolds, including the production of proteolytic enzymes, like matrix metalloproteinases that digest the extracellular matrix [80], and oxygen reactive metabolites which are directly toxic to the cells in the surrounding microenvironment [81], ultimately leading to necrosis and structural tissue damage.
Innate and Adaptive Immune Responses Unique to Pediatrics
Maturation of the mucosal immune system is a continuum with no definitive markers defining a “mature” and “immature” status. The only evidence available from which to draw conclusions unique to the pediatric mucosal immune system is from studies of human neonates or animal studied at the time of weaning. The most noticeable difference between developing (neonatal) and established immune responses would appear to be in adaptive immunity. The rationale for this assumption is that adaptive immune responses are antigen specific and thus require postnatal exposure to dietary and microbial antigen to develop immunologic memory. On the contrary, as pointed out earlier, innate immune responses are for the most part germline encoded through recognition of microbial ligands by pathogen recognition receptors. Indeed, fetal intestinal epithelial cell lines exhibit responsiveness to inflammatory stimuli and bacterial products [82]. Thus, this section will briefly address three established differences in adaptive immune responses between pediatric and adult populations.
Neonates are capable of humoral and cellular immune responses at the time of birth. Immediately upon leaving the birth canal the gastrointestinal tract encounters microbes resident in the birth canal and the surrounding environment. Within hours the gastrointestinal tract is colonized with facultative and strict anaerobic bacteria. Specific secretory IgA responses to organisms such as Escherichia coli are produced within the first week of life [83, 84]. Infants and young children are capable of generating the full spectrum of functional T cells (Th1, Th2, etc.) and T cell-dependent B cell responses; [85] yet, T cell-independent B cell immune responses do not reach full maturity for several years. It is possible that this impaired B cell response to T cell-independent antigens (i.e., polysaccharides) that leaves young children susceptible to encapsulated bacteria (i.e., Haemophilus influenzae type b) [86]. The second major difference is the tendency of the young mucosal immune system to generate systemic immune responses to oral antigen. The evidence for this exists in epidemiologic studies of neonatal protein intolerance [87] as well as animal data demonstrating antigen feeding and the induction of systemic antibody responses [88]. The third point commonly highlighted is the propensity of neonatal effector T cell responses to be preferentially Th2 polarized [89]. The significance of this observation is unclear, but it has been suggested that this may represent a “default” pathway aimed at keeping inflammatory reactions controlled at an early stage of life when vigorous Th1 polarization could lead to damaging inflammatory responses [90]. How any of the three aforementioned neonatal adaptive immune features relate to human IBD remains to be explored.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


