Erika F. Fernandez Key points Since the last edition of this chapter, studies have continued to observe higher blood pressure and less inotrope need in infants given corticosteroids. However, despite the growing number of studies and the increasing utilization of corticosteroids in newborns,1–5 there remains no consensus on a singular pathway for recognition, treatment, and the assessment of best practices and outcomes. This is in comparison to other populations whose societies or colleges have created unified guidelines. The American College of Critical Care Medicine updated their guidelines in 2017 on the hemodynamic support of pediatric and neonatal septic shock.6 In the same year the Multispecialty Task Force for the Society of Critical Care Medicine and the European Society of Intensive Care Medicine updated their guidelines on critical illness–related corticosteroid insufficiency (CIRCI) in critically ill patients.7 Since the initial publications of these guidelines, there has been ongoing testing of these guidelines resulting in ongoing changes and improvements.8 In the newborn population there are no unified standards. The American Academy of Pediatrics (AAP) Committee on Fetus and Newborn (COFN), a leader in proposing standards of care in the United States, recently published its first clinical report in 2022, on the Recognition and Management of Cardiovascular Insufficiency in the Very Low Birth Weight Newborn.9 However, the section on cardiovascular use of steroids is limited, with no clear trigger or strategy for initiating and administering steroids. The last Cochrane Review on Treatment for Hypotension in Very Low Birth Weight Infants was published in 2011, and corticosteroids were recognized to be as effective as dopamine in treating refractory hypotension but, because long-term safety and benefit data were lacking, they were not recommended for routine use.10 Corticosteroid treatment in newborn infants for hypotension has been recommended by various experts in general article reviews.11–14 In one of these reviews Dr Watterberg proposed one of the most detailed algorithms for use of hydrocortisone for hypotension and included suggested dosing and timing.11 However, it remains that without a formally established consensus, it is difficult to assess the extent to which these recommendations have been utilized. There have been significant research efforts to better understand the use of corticosteroids in the newborn population; however, large randomized controlled trials (RCTs) designed to evaluate short and long-term effects of hydrocortisone for the treatment of cardiovascular insufficiency are very challenging to conduct and execute. The Neonatal Research Network has attempted to conduct RCTs on the hemodynamic effects of corticosteroids in sick preterm and term infants with hypotension in the past decade but was unable to complete the enrollment of patients.15,16 Reasons for the low enrollment rates included fewer-than-anticipated infants with low blood pressure, difficulties in obtaining consent within the narrow study window, and lack of physician equipoise. In addition, many patients at the time of screening (within 2–12 hours of age or onset of presentation) have already been administered corticosteroids (up to 33% and higher).16,17 The enrollment of preterm infants has also been hampered by high rates of early indomethacin administration. With the difficulty of conducting rigorous studies and given the use of steroids in the acutely ill newborn population, alternative approaches and creativity may be needed to answer the question of best clinical practices, long-term safety, and potential benefits. There is a need to construct a clear and consensus-driven guideline directing a more standardized approach to the use of corticosteroids in the newborn infant. This should include: (1) triggers to aid recognition of infants who may be amenable to corticosteroids, including clinical presentation, disease states, and laboratory values; (2) a pathway for the administration of corticosteroids, including type, timing, dosing, and duration; and (3) a standardized assessment of performance of the pathway to evaluate safety and overall outcomes. The most common clinical presentation in the newborn infant who may be responsive to corticosteroids is hypotension and, in particular, those with “vasopressor-resistant hypotension”. Vasopressor-resistant hypotension is present when hypotension persists despite vasopressor administration. There are varying definitions of vasopressor-resistant hypotension based on varying inotrope levels but it is most often defined as when dopamine dose is at least >10 mcg/kg/min. Dopamine is usually considered the first line for treatment of hypotension because, despite the fact that small studies showed no difference between hydrocortisone and dopamine as first-line treatment in morbidity or mortality, it is the most studied.18 Hypotension in the critically ill newborn has been associated with adrenal insufficiency. However, regardless of the presence of adrenal insufficiency, corticosteroids elevate systemic blood pressure, so it is important to understand the populations and disease states in which corticosteroids have been studied. Until there is more information on the effect of corticosteroids on developing organs and their safety, corticosteroids should be limited to those infants requiring “rescue” treatment for vasopressor-resistant hypotension versus prophylaxis. The clinical presentation of infants with adrenal insufficiency often includes hypotension but may also include other signs and symptoms of classic adrenal insufficiency, such as hypoglycemia, hyponatremia, hyperkalemia, acidosis, tachycardia, and weakness (Figure 23.1). However, these signs are often masked by the tightly controlled fluid, electrolyte, and temperature management of infants in the newborn intensive care unit. While this chapter focuses mainly on infants with transient medical conditions which may be responsive to short-course corticosteroids, the clinical presentation may look the same as for those infants with chronic conditions of adrenal insufficiency. An example of a chronic condition is congenital adrenal hyperplasia which requires an extensive workup and will need long-term glucocorticoid replacement.19,20 Fortunately, these chronic diseases are rare (10–15 per 100,000 for chronic primary adrenal insufficiencies and 150–280 per million for central adrenal insufficiencies). However, it is important not to miss these diagnoses and, when faced with a critically ill newborn with vasopressor-resistant hypotension, it may be prudent to obtain a cortisol level prior to initiating corticosteroids to help guide the potential future evaluation for these chronic conditions. Although a singular random cortisol value is often of limited use in these chronic conditions, a random cortisol value >18–22 mcg/dL most often rules out most chronic conditions of adrenal insufficiency, while those with cortisol values <5–7 mcg/dL in critical illness may prompt further evaluation including, at the minimum, an adrenocorticotrophin hormone stimulation test. Relative adrenal insufficiency is characterized by its transience because most patients who recover will have normal HPA axis function and corticosteroid activity. Relative adrenal insufficiency is defined as a condition when there is inadequate corticosteroid activity compared with the level of illness in a critically ill patient7,21–24 and may arise from inadequate cortisol levels resulting from problems anywhere along the HPA axis from synthesis to function or tissue resistance to corticosteroids. Adrenal insufficiency in critically ill patients is also known as “functional adrenal insufficiency”, “transient adrenocortical insufficiency of prematurity”, or “critical illness-related corticosteroid insufficiency”. For the purposes of this chapter, the term “relative adrenal insufficiency” is used interchangeably with these terms. The diagnosis of relative adrenal insufficiency in a critically ill patient should not make one complacent about, or underestimate, the potentially life-threatening nature of this type of adrenal insufficiency.25 Since the 1980s, relative adrenal insufficiency has become increasingly recognized in sick premature and term neonates, infants, children, and adults.7,14,26–51 The overall incidence of relative adrenal insufficiency in critically ill adult patients is approximately 10–20% in general and around 60% in patients with severe sepsis, and septic shock in particular.52,53 Reported proportions of critically ill pediatric patients with “inadequate” cortisol response vary widely, from as low as 2% often up to 87%.43–45,54–56 Overall, in the critically ill neonate the incidence is less well understood because there is no consensus on the diagnostic criteria of relative adrenal insufficiency of the ill newborn.40,43,46,57–68 However, it has been reported that almost 20% of ill, mechanically ventilated late preterm and term newborns and up to 33% of all extreme preterm newborns in the United States are receiving corticosteroids for cardiovascular insufficiency.1,2 The timing of presentation of relative adrenal insufficiency is most often reported in the first few postnatal days in critically ill newborn infants. There is some evidence from a Japanese cohort that adrenal insufficiency can also occur later in life (4–7 days of age) in ill newborn infants with respiratory distress or sepsis.45 Surveys in Japan found that approximately 4–8% of VLBW infants are receiving postnatal corticosteroid therapy after 7 days of age, with symptoms suggestive of relative adrenal insufficiency, including hypotension and high cortisol precursors.69,70 Postulated pathophysiologic mechanisms for relative adrenal insufficiency in ill patients include adrenergic receptor insensitivity due to receptor downregulation (Figure 23.2), proinflammatory cytokine-mediated suppression of the function of the pituitary and adrenal glands, inadequate HPA axis response to stress, limited adrenal reserve, gestational age–associated immaturity of the adrenal gland, corticosteroid tissue resistance, and limited adrenal perfusion (Figure 23.3).32,41,42,71–75 Prolonged exposure to inflammatory mediators is one of the proposed mechanisms for both vasopressor-resistant hypotension manifesting via receptor downregulation and relative adrenal insufficiency presenting with suppression of the function of the adrenal gland and/or HPA axis. Decreased vascular responsiveness to adrenergic agents is due to downregulation of adrenergic receptors.76 Downregulation of adrenergic receptors in clinical conditions occurs within hours due to prolonged exposure to intrinsic (stress response) or extrinsic (vasopressor therapy) catecholamines and inflammatory mediators such as nitric oxide (NO), tumor necrosis factor, and other inflammatory cytokines (interleukin-1 [IL-1], IL-2, IL-6, interferon-gamma).58,77 Thus production of proinflammatory cytokines also contributes to decreased vascular reactivity to catecholamines and thus to the development of vasopressor-resistant hypotension and has been described in patients with septic shock.78 In the scenario in which severe illness promotes inflammation and concomitant cortisol insufficiency–associated decreased cardiovascular responsiveness to catecholamines, corticosteroid therapy re-establishes vascular responsiveness and counteracts inflammation. Newborns, especially those born prematurely, have unique susceptibilities to relative adrenal insufficiency, which is, at least in part, due to the immaturity of their HPA axis and especially the cortical function of the adrenal gland. In addition, the changes in adrenal cortical hormone production, especially cortisol during the transition to extrauterine life and the gestational age–dependent changes in placental 11β-hydroxysteroid dehydrogenase type 2 activity, and thus the effect of maternal corticosteroids on fetal corticosteroid production, pose special challenges for the newborn, especially the preterm neonate. Fetal adrenal glands begin to synthesize cortisol de novo at approximately 22–24 weeks’ gestation, followed by a steady increase throughout the rest of the pregnancy.79 Corticotrophin-releasing hormone (CRH) production by the placenta also increases through gestation, resulting in maternal and fetal serum CRH concentrations at term that are much higher than at any other time in life.80 Placental CRH stimulates cortisol production in the fetal adrenal gland. At birth, the very high placental CRH production ceases to have an effect on the newborn. The pituitary gland of the newborn, which has been exposed to high concentrations of CRH during fetal life, may become transiently insensitive to the lower concentrations of CRH produced by the hypothalamus of the neonate. Therefore it may not be able to increase ACTH production to appropriately stimulate the adrenal gland for several days after delivery. Healthy term newborns tolerate this period of relative HPA insufficiency. However, this situation may predispose the newborn to the development of relative adrenal insufficiency in critical illness. Relative adrenal insufficiency could then contribute to the severity of systemic hypotension and attenuate the response of the cardiovascular system to treatment. Relative adrenal insufficiency has been described in ill preterm infants who present with cardiovascular instability in addition to random and/or stimulated cortisol levels that are inadequate for the degree of illness severity and by rapid clinical and hemodynamic improvement following corticosteroid therapy.23,46,51,52,54 In 1989, Ward and Colasurdo were the first to describe a small cohort of ventilated, sick, extremely premature infants who presented with signs consistent with adrenal insufficiency or “Addisonian crisis”.28 These infants had signs of relative adrenal insufficiency, including hypotension, oliguria, and hyponatremia and had cortisol values less than 15 mcg/dL (414 nmol/L). Since this time, subsequent investigations have further elucidated the clinical and laboratory presentation of relative adrenal insufficiency in ill, hypotensive premature infants.27–30,33,34,37,39,45,47,81–86 The study by Yoder and colleagues in very premature baboons has provided the most direct, albeit non-human, evidence that relative adrenal insufficiency, cardiovascular insufficiency, and prematurity are related.87 In earlier studies, this group of investigators documented that most extremely premature baboons, delivered at approximately 67% of baboon gestation (around 26 weeks’ human gestation), required volume expansion and vasopressor-inotrope therapy to treat the subjects’ hypotension, oliguria, and acid-base imbalance. Many of the premature baboons (38%) also required hydrocortisone to successfully treat their hypotension despite receiving vasopressor-inotrope therapy.88 This group of researchers also demonstrated that decreased urinary free cortisol excretion in the first postnatal day correlated with decreased left ventricular function. Furthermore, hydrocortisone therapy (0.5–1.0 mg/kg/day for 1–2 days) corrected the hypotension and the left ventricular dysfunction, reduced vasopressor-inotrope requirement and mortality, and increased serum cortisol to levels comparable to those seen in baboons with no evidence of relative adrenal insufficiency or cardiovascular dysfunction.87 Investigators have documented two intriguing observations regarding cortisol levels in well and sick premature infants. Many healthy premature infants, with no signs of relative adrenal insufficiency, have random cortisol levels that are not detectable or less than 5 mcg/dL (138 nmol/L), a threshold considered to indicate adrenal insufficiency.29,35,36,40,47,54,82,84–86,89–91 On the other hand, there is a population of sick premature infants with serum cortisol levels similar to or lower than cortisol levels in well preterm or term infants.27–29,36,54,84,85,90,92,93 The finding that sick premature infants do not have the expected increase in random cortisol levels commensurate with their illness acuity is supportive of the presence of relative adrenal insufficiency in this population. Relative adrenal insufficiency has been described more often in those ill preterm infants whose predominant clinical presentation is hypotension. Blood pressure has been found to positively correlate with the cortisol production rate,94 where patients with low random or basal serum cortisol values were more likely to have low blood pressure. In an RCT of hydrocortisone administration in hypotensive preterm infants, the overall baseline (control) median serum cortisol concentrations were 3.3 and 4.1 mcg/dL in the treated and placebo groups, respectively.54 These values are considered low for the degree of illness severity in preterm infants when compared to the 50th percentile of the serum cortisol values in ill infants (7.2 mcg/dL).4 In 54 surfactant-treated, 1-day-old preterm infants of 24–36 weeks’ gestation, serum cortisol values were lower in those with left ventricular output ≤180 mL/min/kg compared to those >180 mL/min/kg.30,48,95 Further support for relative adrenal insufficiency in the preterm population comes from studies that have found that adrenal insufficiency results from immature cortisol synthesis in the adrenal gland. Ng et al. reported normal pituitary response to CRH, but blunted adrenal response, in sick premature infants with vasopressor-resistant hypotension and low serum cortisol values. These findings led Ng et al. to speculate that, in preterm neonates, the adrenal gland was responsible for the adrenal insufficiency.39 Findings from other investigators support this notion because sick premature infants have been found to have low random cortisol levels, elevated cortisol precursors, and blunted response to ACTH stimulation.40,47,84,85,90,91,93,96 Watterberg et al. reported that, compared with term infants, sick premature infants had higher cortisol precursor concentrations (17α-OH pregnenolone, 17α-OH progesterone, and 11-deoxycortisol) and lower serum cortisol concentrations. In another small prospective study of infants born at less than 30 weeks’ gestation, Huysman et al. demonstrated that critically ill, ventilated infants, compared with less sick, non-ventilated infants, had lower cortisol levels, elevated cortisol precursor levels of 17-hydroxyprogesterone, and insufficient cortisol response to ACTH (0.5 mcg/kg) stimulation.82 Korte et al. also reported an abnormal adrenal response to cosyntropin (ACTH) in 51 ventilated, premature infants of less than 32 weeks’ gestation who had baseline cortisol levels of less than 5 mcg/dL (138 nmol/L). Among the 51 infants, 64% and 37% had inadequate cortisol response (<9 mcg/dL) to stimulation with 0.1 and 0.2 mcg/kg cosyntropin, respectively.40 Preterm infants, due to immaturity of the HPA axis, may be at unique risk for adrenal insufficiency and may be considered candidates for corticosteroids when not responsive to otherwise routine management, including volume and vasopressors. There is growing evidence that many ill term and late preterm infants also exhibit evidence of relative adrenal insufficiency.44,45,56,89,97–99 In 1972, Gutai et al. were one of the first groups of authors to describe suboptimal cortisol responses to illness in a small number of stressed term newborn infants.100 Indeed, there was no difference in the median random cortisol value between the 12 ill infants (5.2 mcg/dL) and 28 healthy newborns (4.1 mcg/dL) but all infants responded to 5 units of ACTH appropriately. A subsequent study by Thomas et al. found that 27% of ill term newborns studied had a basal cortisol of less than 2 mcg/dL, and only 33% of these infants had an appropriate response to ACTH (>18 mcg/dL).89 In the first study to investigate cardiovascular responses to dexamethasone in hypotensive term newborn infants, Tantivit et al. reported that five of the seven infants studied had cortisol values <10 mcg/dL.55 All of these infants responded to dexamethasone administration with prompt hemodynamic stabilization. Soliman et al. found an overall increase in basal circulating cortisol concentrations by two- to threefold in neonates with sepsis and respiratory distress.45 Yet, over 30% of these infants had cortisol values suggestive of relative adrenal insufficiency (<15 mcg/dL). Patients with lower basal cortisol levels and peak cortisol responses to ACTH had higher mortality. Recent reports also suggest that, similar to preterm infants, critically ill late preterm and term infants with hypotension may have problems with adrenal synthesis of cortisol, as higher cortisol precursor levels have also been documented in those presenting with vasopressor-resistant hypotension.51,101 In a larger prospective observational study 35 sick late preterm and term infants on mechanical ventilation had a median random cortisol level of 4.6 mcg/dL, which is a value very low for the degree of illness.56 These infants had relatively low ACTH values as well but demonstrated appropriate cortisol responses to ACTH stimulation (1 mcg/kg), suggesting this study population had secondary adrenal insufficiency. Recognizing that acutely ill term and preterm infants may be at risk for adrenal insufficiency due to developmental reasons, including prematurity of the HPA axis at varying levels or due to poor/delayed transition of the HPA axis immediately after birth, may help guide recognition of infants who may be considered for steroid treatment. Subsets of these ill infants with acute pulmonary hypertension (aPH), meconium aspiration syndrome (MAS), hypoxic-ischemic encephalopathy (HIE), and sepsis have undergone further study with regard to their responsiveness to glucocorticoids and/or the presence of adrenal insufficiency. There has been increasing attention on the effect of corticosteroids in those infants with aPH. Corticosteroids have been shown to improve oxygenation by decreasing phosphodiesterase-5 (PDE5) expression and activity and increasing cGMP, thereby promoting vascular dilatation of the lungs in animal models (lambs) with PH.102 One small study enrolled newborns with aPH due to all causes and found that in 15 patients, those who received intravenous hydrocortisone had an increase in systemic blood pressure and an improvement in oxygenation measures.5 Cortisol levels were not measured, so it was unclear if infants were responsive due to relative adrenal insufficiency or to the other basic physiologic effects of corticosteroids such as the effect on inflammation. In one of the largest observational cohort studies, 30% of the 2743 infants with aPH including those with MAS and congenital diaphragmatic hernia (CDH) had received hydrocortisone.5,103 A comparison between the hydrocortisone-treated group and the non-treated group, however, showed no difference in outcomes of death, chronic lung disease (CLD), or oxygen at discharge. In a small subset of these infants with MAS there was lower oxygen use at discharge (odds ratio [OR] 0.56, 95% confidence interval [95% CI] 0.21–0.91). Cortisol values were not measured. In infants with MAS, studies have more often focused on the administration of steroids for the effect on inflammation due to the presence of meconium in the lungs versus the presence of adrenal insufficiency or hypotension.104 In a small study term infants born with meconium-stained amniotic fluid who presented with respiratory distress had lower ACTH and cortisol levels than did infants with meconium-stained amniotic fluid without respiratory distress.105 There has been recent interest in infants undergoing hypothermia treatment for HIE with volume-resistant hypotension. An RCT of 35 infants randomized to receive hydrocortisone of 0.5 mg/kg/dose q 6 hours versus placebo had low cortisol values suggestive of adrenal insufficiency (median 3.5 mcg/dL in treatment group and 3.3 mcg/dL in placebo group). The infants who received hydrocortisone more often reached the target blood pressure in 2 hours after hydrocortisone (94 vs. 58%) and required less inotrope duration and dosage compared to the placebo group.106 Another similar study of 30 infants with HIE found similar cortisol levels between those with vasopressor-resistant hypotension versus those without, but higher DHEA levels in those with hypotension, suggesting adrenal insufficiency.107 In pediatric patients with sepsis the debate continues whether corticosteroids should be administered mostly because of the lack of large RCTs to support the evidence. However, surveys show that 90% of clinicians already agree that there are certain septic patients (those with vasopressor-resistant hypotension) who may benefit from corticosteroids.17 Newborns may be at higher risk due to the immaturity of the HPA axis to synthesize cortisol soon after birth, in addition to the potential HPA axis changes due to inflammation from sepsis. Studies have shown there are lower-than-expected cortisol levels, elevated precursors to cortisol, and very high DHEA levels in septic newborn infants with therapy-resistant hypotension.50,108 One of these studies by Bhat et al. showed that 17 of 30 infants (57%) with neonatal shock had cortisol values ≤15 mcg/dL with adequate rise (≥9 mg/dL) after ACTH, suggesting poor response to the illness and adequate response to ACTH, but with depressed baseline levels.50 In 2000 Pittinger et al. first described low cortisol values in sick infants with CDH and found that 79% had random cortisol levels of <7 mcg/dL.99 Two of the four critically ill patients had an inappropriately low cortisol response to cosyntropin. However, a recent study in which up to 34% of infants received hydrocortisone found that infants exposed to a longer course of hydrocortisone had an increased risk of sepsis (OR 1.04, 95% CI 1.005–1.075, P = 0.02) and mortality (OR 1.11, 95% CI 1.02–1.2, P = 0.02).109 Investigators continue to explore the diseases and clinical presentations of infants who may benefit from corticosteroid treatment while continuing to assess for the potential complications of treatment. Although there are newborn patients whose clinical presentation and disease suggest they may benefit from adjunctive corticosteroid administration, there is still not a reliable mechanism by which to identify these patients. Measuring adrenal function can be complex because acute illness may affect the HPA axis at varying levels and also varies with stage of development, age, disease state, and timing of the disease. The diagnosis of relative adrenal insufficiency is made by determining “inadequate” cortisol response or cortisol production and has been defined in various ways. In other ill populations there are recommended guidelines for diagnosing relative adrenal insufficiency. The recommendation for critically ill adults was proposed in 2008 as a total serum cortisol level of less than 9 mcg/dL following 250 mcg of cosyntropin (ACTH) administration or a random total cortisol of less than 10 mcg/dL (276 nmol/L).110 The updated guidelines in 2017, curiously, showed less enthusiasm for these thresholds but they remained the same.7 In their most recent 2017 publication on clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock, the American College of Critical Care Medicine has changed their stance from 2007. In 2007, it was stated that there was “equipoise on the question of adjunctive steroid therapy and thus the diagnosis of relative adrenal insufficiency for pediatric and newborn septic shock (outside of classic adrenal insufficiency) is pending further trials”. In the new 2017 guidelines, the recommendation is to treat a subset of infants with septic shock and vasopressor-resistant hypotension with hydrocortisone who have absolute adrenal insufficiency as defined by peak cortisol <18 mcg/dL after ACTH or basal cortisol <4 mcg/dL or basal cortisol <18 mcg/dL with need for inotropic support.6,68 Devising diagnostic criteria for relative adrenal insufficiency in infants is particularly challenging because of the relatively little amount of available data.111 Neonatal investigators most often report isolated, random serum cortisol levels or stimulated serum cortisol levels at specific time points following ACTH stimulation. Using random cortisol values at the time of stress in VLBW infants, Korte et al. were some of the first investigators to define normal adrenal function as a random cortisol level of >15 mcg/dL (414 nmol/L) and an inadequate serum cortisol as a serum cortisol level less than 5 mcg/dL (<138 nmol/L).40 In critically ill term and late preterm infants Fernandez et al. showed that those with random cortisol values less than 15 mcg/dL had higher blood pressure within 24 hours after hydrocortisone administration than those with cortisol values >15 mcg/dL.44 In addition, these infants demonstrated a decreased need for vasopressors and had a lower heart rate after hydrocortisone administration. In term and late preterm infants, a cut-off random cortisol value of 15 mcg/dL is often used, based on smaller studies and the pediatric and adult population, or 18 mcg/dL in the presence of vasopressor-resistant hypotension.6,14 In his review, PC Ng published a mathematical model that further takes into account clinical variables that may change the percentile of the diagnostic serum cortisol for preterm infants. He suggested that a serum cortisol value of less than 200 nmol/L (7.25 mcg/dL) be used to diagnose relative (transient) adrenal insufficiency of prematurity. This value represents the 25th percentile of serum cortisol levels in well VLBW and the 50th percentile in vasopressor-dependent VLBW infants.14 However, debate continues on the use of random cortisol values as a diagnostic tool because total plasma cortisol can have marked hourly variability in both adults and neonates.53,112,113 In addition, random cortisol values may not correlate with outcome, response to therapy, or severity of illness.56,112,114 However, a baseline random cortisol is often the most practical and easiest to obtain in the acute setting of the rapidly deteriorating newborn and may help to determine the need for further evaluation. Of note is the fact that cortisol level thresholds may also change slightly based on the laboratory methods. Newer laboratory methods of mass spectrometry and platform methods (Elecsys Cortisol II, Roche Diagnostics, Mannheim, Germany) are more specific because they can detect lower cortisol concentrations than older standard immunoassays.115 The newer methods tend to show lower cortisol values by 1–2 mcg/dL compared to the older ones. To further test for relative adrenal insufficiency, investigators have more often used the ACTH stimulation test as opposed to CRH alone.92,93,116 ACTH test is done by using the corticotropin analogs such as tetracosactrin (Synacthen) or cosyntropin (Cortrosyn). ACTH as the stimulating agent has been trialed with dosages ranging from 0.1 to 62.5 mcg/kg.36,40,45,60,82,84,89,93,117–120 Adrenal stimulation with supraphysiologic ACTH doses can induce a compromised adrenal gland to produce an “adequate”-appearing serum cortisol response and thus miss the diagnosis of relative adrenal insufficiency.40 Indeed, ACTH doses at 0.1–0.2 mcg/kg40,60,117,118 and doses at 0.5–1.0 mcg/kg60,82 are more likely to reveal “relative” adrenal insufficiency than higher ACTH doses. Yet, even within the dose range of 0.1–1.0 mcg/kg, the proportion of sick premature infants with “inadequate” cortisol response varies greatly. In a study of ventilated VLBW infants of less than 32 weeks’ gestation and with random serum cortisol levels of less than 5 mcg/dL (138 nmol/L), Korte et al. reported that 64% and 37% of infants had inadequate cortisol response (less than 9 mcg/dL) to ACTH at doses of 0.1 and 0.2 mcg/dL, respectively.40 In a trial of ventilated 3-week-old 25 weeks’ gestation preterm infants, Watterberg et al. reported that 21% and 2% of infants had inadequate cortisol response to 0.1 and 1.0 mcg/kg ACTH, respectively.60 In critically ill term infants, after giving 1 mcg/kg of ACTH, Fernandez et al. found no infants with a cortisol value of less than 18 mcg/dL, and all patients had increased serum cortisol by greater than 9 mcg/dL.56 In addition, there was no association between ACTH-stimulated cortisol values and severity of illness, need for vasopressor-inotropes, or days on mechanical ventilation. In non-mechanically ventilated infants with sepsis, Soliman et al. found that when an ACTH stimulation dose of 250 mcg/1.73 m2 was used, no patients met the criteria of having adrenal insufficiency, whereas 13% of the patients were diagnosed with adrenal insufficiency when 1 mcg/1.73 m2 of ACTH was given.45 Importantly, patients with lower stimulated cortisol values did have higher mortality. In 2012, Hochwald et al. reported that if an ACTH stimulation test using a dose of 1 mcg/kg is performed in premature infants of less than 29 weeks’ gestation, with varying degrees of illness in the first 8 postnatal hours, the test could predict those with adrenal insufficiency using hypotension requiring vasopressor-inotrope treatment as a marker.121 They determined that an ACTH-induced change in cortisol of less than 12% from baseline provided the highest sensitivity (75%) and specificity (93%) for detecting the development of hypotension in this population. On the other hand, random basal cortisol levels did not predict hypotension with an area under the receiver operating characteristic curve (ROC) of 48%.121 The most recent standard for performing the ACTH stimulation test is to use a total dose of 1 mcg of ACTH (1 mL Cortrosyn diluted in 1- or 2-mL normal saline) and administer over 2 minutes, after which the IV is then flushed. Cortisol levels are measured at baseline and then at 30 minutes. Measuring cortisol levels additionally at 60 minutes after ACTH may reduce the risk of false-positives. Cortisol thresholds for a normal ACTH stimulation test are in the range of >15–19 mcg/dL, but most often it is >18 mcg/dL.122,123 Measuring ACTH, unless undergoing evaluation for chronic adrenal insufficiency, is not feasible in most institutions and there is usually not a sufficient turnaround time to have an impact on the acute management of the critically ill patient. During critical illness, plasma corticotropin levels have also been variably found to be low, normal, or high and likely follow a dynamic pattern over the course of the disease and vary with developmental age.7 Free cortisol and not protein-bound cortisol is responsible for the physiologic effects at the cellular level and was thought to potentially be a better diagnostic tool for adrenal insufficiency.125 Yoder et al. found that urinary free cortisol levels were directly proportional to and highly correlated with plasma cortisol levels in premature baboon models.124 Urinary free cortisol measurements may also avoid the potential fluctuations seen in serum cortisol levels and the need for frequent blood sampling.87 Vezina et al. described unbound cortisol concentrations in critically ill newborns with hypotension requiring vasopressor-inotrope administration.125 However, free cortisol levels have not been fully validated in the ill newborn populations. Additionally, recent reports suggest that free cortisol levels may also not be added information in ill children with septic shock.126 In addition, free cortisol tests are not always widely available and the time to get results is often too long to impact decision-making. The diagnosis of adrenal insufficiency in the preterm and term neonate, according to measurements of serum cortisol and the response to ACTH stimulation, may be influenced by the various procedures and the testing itself in acutely ill patients.127 Thus the validity and interpretation of adrenal function tests, especially amid critical illness, is subject to an ongoing debate among adult, pediatric, and neonatal critical care physicians. However, there are general upper and lower threshold cortisol values that may help to guide decisions. Sometimes, cortisol values may not help identify those patients who might benefit from corticosteroids as there are conflicting reports on the correlation of cortisol levels with response to therapy or outcomes. There are a number of reports that also show documented low cortisol values in neonates with vasopressor-resistant hypotension responsive to corticosteroid treatment.27,28,30,33,39,54,83,128 However, other studies report a similar presentation of responsiveness to corticosteroids treatment without cortisol data or relation to cortisol data.30,33,45,129,130 Hydrocortisone is by far the most widely used over dexamethasone, both clinically and in studies, likely due to its better safety profile and the additional mineralocorticoid activity. Studies of early use (<7 days of age) and higher-dose dexamethasone demonstrate an adverse effect on neurodevelopment in preterm neonates.120,131 Supportive evidence from animal and laboratory studies indicates that unbalanced stimulation of glucocorticoid receptors in the brain induces apoptosis, which can be rescued by the addition of a mineralocorticoid.132 This would explain why dexamethasone, especially when given early and in higher doses (0.5 mg/kg q 12 hours 3 times during the first 2 postnatal days),133 is associated with significant cerebral pathology (white matter injury, decrease in brain volume, etc.), whereas hydrocortisone seems to be devoid of such effects. Hydrocortisone increases blood pressure and reduces vasopressor requirements in all populations. A meta-analysis in preterm neonates with hypotension and vasopressor dependence found that hydrocortisone is effective in increasing blood pressure (seven studies, N = 144, r = 0.71, 95% CI 0.18–0.92) and reducing vasopressor requirement (five studies, N = 93, r = 0.74, 95% CI 0.0084–0.96). The study’s findings were robust and found that by random effects meta-analysis, the numbers of new studies needed to cancel out the effects of hydrocortisone on blood pressure increase and the decrease in vasopressor requirement are 78 for blood pressure increase and 47 for reducing vasopressor requirement.134 Studies of vasopressor-dependent, critically ill newborn infants have used variable hydrocortisone dosing (1–3 mg/kg/day), and all have shown an increase in blood pressure, a decrease in volume and vasopressor-inotrope requirement, and a decrease in heart rate without confirmed increases in adverse events.30,37,44,54,114,134,135 In preterm infants doses as low as 1 mg/kg/day of hydrocortisone have been shown to increase blood pressure significantly.119 A recent study of 106 preterm infants with hypotension on inotropes showed no differences in efficacy between dosing of 4 mg/kg/day versus 1–3 mg/kg/day.136 A planned systemic and meta-analysis has been proposed to study different doses of hydrocortisone on the effect of end organ perfusion in infants with hypotension: low-dose (initial dose of ≤1 mg/kg, followed by ≤2 mg/kg/day or cumulative daily dose of ≤3 mg/kg on the first day of treatment) versus high-dose (initial dose of >1 mg/kg, followed by >2 mg/kg/day or cumulative daily dose of >3 mg/kg on the first day of treatment).137 The elimination half-life of bound hydrocortisone in neonates is much longer than in adults. In adults the half-life of bound cortisol is reported to be 2 ± 0.3 hours138 and, for free cortisol, 61 minutes.139 In 1962 Reynolds et al. were the first to report pharmacokinetics in five term infants after a bolus of 5 mg/kg of hydrocortisone.140 The mean peak serum concentration (30–80 minutes after dose) was 557 mcg/dL, and mean serum half-life was just under 4 hours (range 2.4–7.1 hours). In a recent study of unbound hydrocortisone concentrations in 62 preterm infants treated for vasopressor-resistant hypotension, the half-life of free cortisol was 2.9 hours.125 This is three times longer than in the adult population and consistent with other reports on hydrocortisone pharmacokinetics in preterm infants as described in a recent review.11 As illustrated in this review, high cortisol levels can result from administration of high or “stress” dosing of hydrocortisone because the longer half-life in preterm infants is often not appreciated. In a small pilot study of six infants hydrocortisone at 0.4–0.8 mg/kg/dose resulted in serum cortisol trough levels greater than 20 mcg/dL for 8 to 12 hours compared to the median endogenous serum cortisol value in sick extremely-low-birth-weight (ELBW) infants of 16 mcg/dL at less than 48 hours of age. Given this information, hydrocortisone should be given at no shorter interval than every 6 hours for late preterm and term infants and up to every 12 hours in extreme prematurity. The time to documented cardiovascular response to hydrocortisone is often measured in hours in the ill newborn population. In preterm infants, Helbock reported an increase in blood pressure as early as 30 minutes and within 2 hours following hydrocortisone therapy (1 mg) in 25- to 26-week gestation infants with vasopressor-resistant hypotension.83 Gaissmaier and Pohlandt noted improvement in vasopressor-resistant hypotension within 4–8 hours following a single injection of dexamethasone.128 Seri et al.30 and Noori et al.129 reported an increase in blood pressure within 2 hours of hydrocortisone and low-dose dexamethasone (0.1 mg/kg) administration, respectively. Mizobuchi found a similar response after a single dose of 2 mg/kg dose of hydrocortisone.141 Ng et al. found more infants were weaned off vasopressor-inotropes by 72 hours after receiving hydrocortisone vs placebo (79% vs. 33%, P = 0.001).142 In 15 newborn infants Noori et al. studied the hemodynamics following hydrocortisone (2 mg/kg, followed by 1 mg/kg q 12 hours) and showed an increase in blood pressure at 6 hours, a parallel increase in systemic vascular resistance, and a decrease in dopamine dosage without initial changes in stroke volume or left ventricular output.135 In seven term ill newborn infants given dexamethasone at 0.2 mg/kg/day, an increase in blood pressure was noted by 4 hours after initial dosing, with a concomitant decrease in heart rate and vasopressor dosage.55 Vasopressors were discontinued in all infants within 72 hours of initiating dexamethasone. In a larger study of ill late preterm and term neonates there was a significant change in blood pressure over the first 24 hours after hydrocortisone and an overall decrease in vasopressor dose and heart rate within 12 hours.44 In 12 term infants given hydrocortisone for low cardiac output syndrome after cardiac surgery, the blood pressure increased 3 hours after hydrocortisone.130 Baker et al. also found an increase in blood pressure, starting 2 hours after the first dose of hydrocortisone, and a decrease in the total vasopressor-inotrope requirement at 6 hours in a study of 117 critically ill term and preterm newborns.114 In summary, most newborn infants respond with improved blood pressure within 2–4 hours of initiation of corticosteroids and weaning from inotropes as early as 6 hours after initiation and most by 72 hours. In most infants with possible adrenal insufficiency-associated hypotension, blood pressure recovers in less than a few days after corticosteroid initiation, with few preterm infants requiring longer treatment. This is similar to what is found in acutely ill adults where the reversal of shock, as measured by the timing of withdrawal of vasopressors, occurs earlier after receiving hydrocortisone versus placebo (3.3 vs. 5.8 median days).42 In a group of adults with sepsis and higher illness severity, the median time to vasopressor withdrawal with corticosteroids versus placebo also favored the treated group (7 vs. 9 days, respectively).42 In preterm infants, Colasurdo et al. found that in nine sick infants of 26 weeks’ gestation with clinical symptoms of relative adrenal insufficiency and low cortisol levels (mean 251 ± 102 nmol/L or 9.1 ±3.7 mcg/dL), there was reversal of clinical signs within 2 days. Ng et al. reported that 79% of preterm infants <32 weeks’ gestation with refractory hypotension receiving hydrocortisone for 5 days were successfully weaned from vasopressor support by 72 hours of age compared with only 33% who were receiving placebo.54 Hochwald et al. also showed that a 2-day course of hydrocortisone compared with placebo decreased dopamine exposure by 34 hours versus 67 hours (P = 0.04).143 Similarly, Efird et al. reported that prophylactic hydrocortisone therapy for 5 days (versus placebo) in ELBW infants reduced the use of vasopressors during the first 2 postnatal days.144 On the other hand, in very premature baboons with relative adrenal insufficiency and vasopressor-resistant hypotension at a gestational age approximately equivalent to human gestation of 26 weeks, there was no decrease in urinary free cortisol levels over the first 2 postnatal weeks (the entire duration of the study).87 In term ill infants, Economou et al. found that, in 4 of 15 infants who were “low cortisol responders” relative to their illness, this state lasted until the fifth postnatal day.97 In another study of critically ill newborn infants with refractory hypotension, Baker et al. used cortisol levels and the presence of hemodynamic instability to determine the duration of relative adrenal insufficiency and thereby the duration of hydrocortisone replacement therapy.114 In total, 61 term infants received 3.5 median days of hydrocortisone therapy, whereas 37 extremely preterm infants enrolled in the study remained on hydrocortisone for a median of 15 days. In another study 1-day-old term and late preterm ill infants with random low cortisol values of less than 15 mcg/dL received longer courses of treatment with hydrocortisone for hypotension compared with those with higher cortisol values (5 vs. 2.5 median days).44 Kamath et al. found a high incidence (67%) of cortisol values of less than 15 mcg/dL in hypotensive infants with congenital diaphragmatic hernia, all of whom received hydrocortisone for 10.9 ± 7.0 days.98 The duration of relative adrenal insufficiency varies with age, disease severity, etiology, and response to treatment. In general, relative adrenal insufficiency seems to last less than a week in term infants and no longer than 2 weeks in very preterm infants, irrespective of whether it is defined by cortisol values or by the short-term cardiovascular response to corticosteroid replacement therapy. However, exposure to corticosteroids should be limited as much as possible and most infants appear to have significantly less inotrope exposure after 72 hours. While corticosteroids have continued to show positive effects on stabilizing cardiovascular insufficiency, there remains caution because of the limited data on safety due to the lack of RCTs. It is well known that high-dose corticosteroids in all populations have associated adverse effects. High-dose corticosteroids have been shown to increase mortality and infection rates in septic and ill adults and increase rates of neurodevelopmental delays (i.e., cerebral palsy) in preterm infants.53,145 It is not clear that lower dose hydrocortisone is without potential risk long term. In a recent small study of preterm infants with septic shock, hydrocortisone improved blood pressure, urine output, and oxygen requirement. There was, however, a decrease in 1-year survival in those who received hydrocortisone, suggesting more information is needed.146 In addition, there may be particular populations of infants more at risk of complications due to hydrocortisone such as found in the study by Peeple et al.136 In hypotensive infants on inotropes who received hydrocortisone, those with cortisol values >15 mcg/dL showed less improvement in vasoactive burden, increased hyperglycemia (P = 0.015), and increased death independent of the hydrocortisone dose (OR 26.3, range 3.5–198.3, P = 0.002), suggesting that hydrocortisone therapy should be avoided in infants who are not cortisol deficient. Shorter-term outcomes in newborn infants have shown that both low- and high-dose corticosteroids have been associated with hyperglycemia, hypertension, and hypertrophic cardiomyopathy.147–149 In addition, there have been reports of spontaneous ileal perforations when preterm infants are co-exposed to hydrocortisone or dexamethasone and cyclooxygenase inhibitors (i.e., indomethacin). Despite these potential complications, the data from RCTs on hydrocortisone treatment for the prevention of BPD in preterm infants are reassuring. Hydrocortisone use in preterm infants for the prevention of BPD has shown either positive long-term outcomes (neurodevelopment or mortality) or at least no difference versus placebo.145,148,150,151 Given the pros and cons of using corticosteroids, they should, at the minimum, be considered for patients with fulminant manifestations of critical illness, especially those with volume and vasopressor-resistant hypotension, as there is equipoise to indicate that they may improve outcome with acceptable risks when used at the lowest effective dose and shortest course possible. Clinical, biochemical, and physiologic evidence indicates that adrenal insufficiency and vasopressor-resistant hypotension are serious conditions in sick preterm and term infants and that these conditions respond to corticosteroid therapy, with improvements in cardiovascular status. Adequate HPA axis and adrenal function are vital to postnatal adaptation in extremely premature infants and critically ill late preterm and term newborns. Yet, recent findings provide important insights into adrenal insufficiency and vasopressor-resistant hypotension and stimulate consideration of mechanisms for adrenal insufficiency and vasopressor-resistant hypotension. Management of the critically ill hypotensive infant remains challenging and requires a better understanding of the pathophysiology of neonatal shock and improvements in our ability to monitor cardiac output, organ blood flow, and tissue perfusion in real time at the bedside. In addition, we still need to improve our understanding of the pathogenesis and epidemiology of adrenal insufficiency and vasopressor-resistant hypotension, especially in terms of the determinants, mechanisms, and characteristics of these conditions, and their relationship with each other and with acute and long-term morbidity/mortality. Ultimately, we need to provide evidence that our interventions also improve clinically meaningful outcomes. Another challenge is to improve the diagnostic methods to assess adrenal function and vasopressor-resistant hypotension and define the criteria for treatment. We need to be able to determine which patient needs therapy, at what dose and for how long, and what evaluations provide the most reliable information early in the course of the disease, such as the use of echocardiograms and cortisol values. Are serum cortisol values the optimal measure of adrenal function? Can one improve diagnostic accuracy and prognosis by combining the findings of different tests in conjunction with the status of the patient? Will simultaneous measurements of inflammatory mediators improve our understanding and guide therapy? By improving our ability to establish accurate and timely diagnosis of adrenal insufficiency, will we be able to target high-risk patients for investigational and clinical interventions and avoid unnecessary exposure of lower-risk infants to corticosteroid therapy? More comprehensive issues to be addressed include identifying the factors that influence the choice of corticosteroid treatment and establishing the dosage, duration, and response to therapy of the individual patient.152 We also need to find out whether corticosteroid regimens need to be adjusted according to disease severity or the response to treatment to maximize effectiveness and minimize harm. Scientifically and ethically sound research and carefully designed studies will resolve these questions but answers may also require a different approach than only applying rigorous RCTs. Any approach should include framework for recognition, treatment strategy, and implementation and performance assessment of feasibility, adherence, effectiveness, and outcomes.
Chapter 23: Glucocorticoids and adrenal function in neonates with hypotension
Introduction
Recognition of potential patients for the administration of corticosteroids
Clinical presentation and patient characteristics
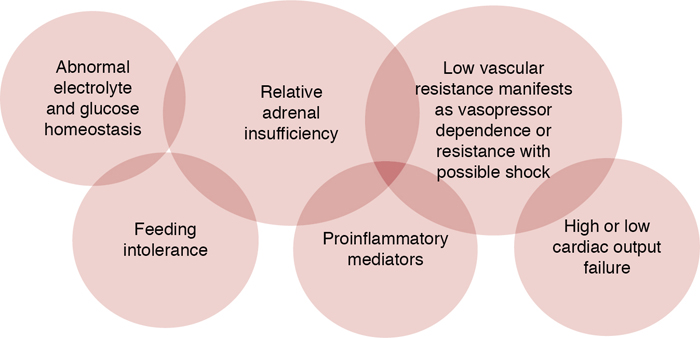
Adrenal insufficiency
Relative adrenal insufficiency
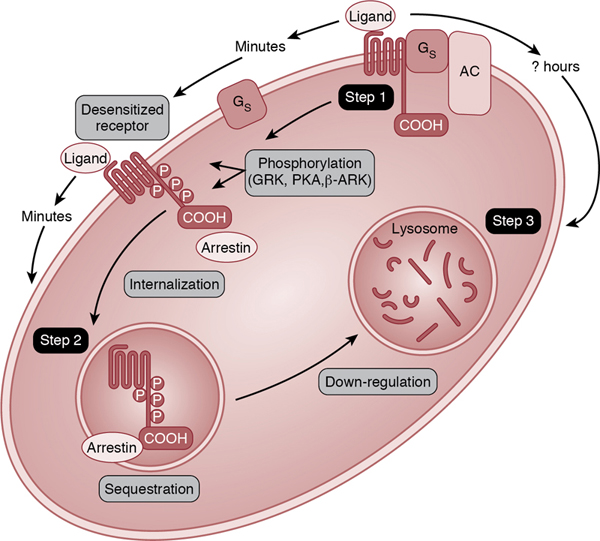
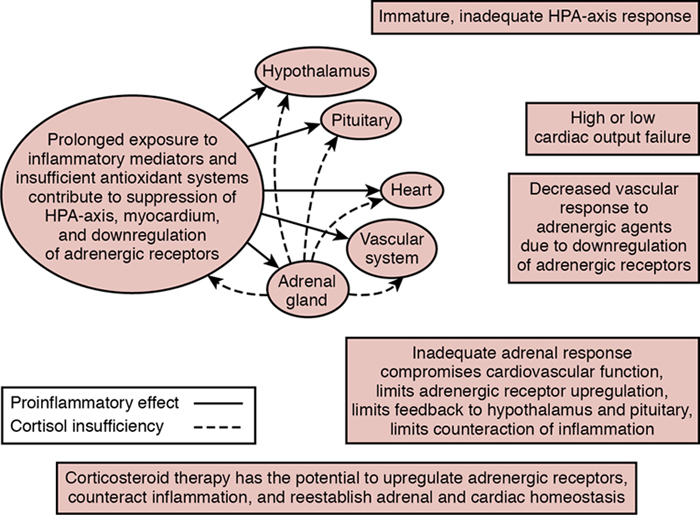
Relative adrenal insufficiency in preterm infants
Relative adrenal insufficiency in late preterm and term infants
Acute pulmonary hypertension (aPH) of newborn
Meconium aspiration syndrome
Hypoxic-ischemic encephalopathy
Sepsis
Congenital diaphragmatic hernia
Laboratory triggers (cortisol levels)
Corticosteroid administration
Type of glucocorticoid
Clinical effect
Dosing
Onset of action
Duration of adrenal insufficiency
Outcomes
Summary
References