Fig. 2.1
Example of procedure room set-up for office-based surgery
An individual or group of individuals specifically dedicated to office procedures should manage the set-up and maintenance of the room itself. This way, a “team” for office procedures is established and is familiar with what needs to be done prior to, during, and after a procedure. This would also ensure that equipment is available, functioning, sterilized (when needed) and ready to go. The office team may include medical assistants (MA) and/or nurses depending on the scope of practice required. If medication is to be given, either orally, intramuscularly or intravenously, then availability of a nurse is recommended. Office managers or other administrative personnel should be included to help ensure appropriate oversight and compliance as needed. Also, one of the team members should be responsible for documentation before, during, and after the procedure to delineate performance of necessary items of the surgical checklist, referenced below.
A physician must always be readily available before and after the procedure as well. This is of primary importance for safety in the office. If a physician is not present in the office, for instance, pre-procedural medications or anesthetics should not be given in case allergic or other related complications were to occur. The physician is the designated “team leader” and must be accountable to the office staff and to the patients. Ideally, the physician will be in the office setting until all the patients who underwent procedures that day safely leave the office.
Equipment
Performance of office-based surgical procedures requires certain equipment be available in order to perform each procedure as well as have specific supplies readily available in case problems or complications occur during the procedure. A basic list of equipment needed to perform any office procedure is listed in Table 2.1. Most gynecology offices will have many of the suggested pieces of equipment available; however, supplies should be checked prior to each procedure, or optimally at the beginning of each week to assure sterilization of needed equipment is undertaken and to prevent mishaps during the procedure.
Table 2.1
Basic office supplies for performance of office-based gynecologic surgery
• Sterile and nonsterile gloves |
• Antiseptic/cleansing solution (such as betadine or baby shampoo) |
• Cotton swabs |
• Pads or fluid drapes (such as chux pads) |
• Speculum (variety of sizes and types) |
• Tenaculum |
• Dilator(s) or dilator probes |
• Specimen containers |
• Basin |
• Appropriate suture |
• Needle driver |
• Scissors |
• Forceps |
• Syringes and appropriate needles for local anesthetics |
• Normal saline |
For the more invasive procedures (hysteroscopy, both diagnostic and operative, global endometrial ablation, hysteroscopic sterilization, cystoscopy, urodynamics, and colposcopy with LEEP), procedure-specific equipment will need to be purchased or leased. While this process will be physician and office dependent, several points must be considered. First, the physician must be certain to be appropriately trained and credentialed in the procedure (see below). Also, the specifics of certain types of equipment (that of a certain brand or manufacturer) will depend upon both physician preference and cost: use of a certain brand at the hospital where the physician operates may be too costly or unavailable for the office so it may be important to see if a more affordable option is available that provides the same level of effectiveness and safety. Maintenance, sterilization requirements, and associated costs also need to be considered. Overall expense to the office practice needs to be evaluated as well, many times in conjunction with an office manager or financial consultant.
When considering which procedure-specific equipment is needed, it is important to decide whether disposable instruments will be used or whether the use of sterilization equipment will be needed for cleaning and sterilization of reusable equipment, especially the hysteroscope or cystoscope. The care and maintenance of instruments must be maintained according to the manufacturer’s instruction manual and serviced by the specific vendor to ensure equipment is maintained properly to maximize its use and shelf life if disposable instruments are not being used. Improper handling or sterilization of the expensive scopes can lead to equipment damage such as condensation of fluid within the lens. Mishandling of light cords or electrical equipment can lead to damage and/or breakage with increased costs for repair or replacement.
If ultrasound is being utilized in the office, either alone or for use with saline infusion sonography, acquisition of necessary ultrasound machines is needed as well as that needed for appropriate disinfecting and sterilization of the transvaginal probe. Image capture and storage also needs to be addressed as well as if appropriate office or ultrasound site credentialing from a national organization such as American Institute of Ultrasound Medicine (AIUM) is desired or required.
While physicians must be appropriately credentialed and trained in the procedure(s) to be performed in the office setting, it is important that the physician and the office team review information about any new procedure or device being used. Stumpf recommends that all members of the team be acquainted with and knowledgable about new equipment (and for some office nurses and medical assistants, hysteroscopy and cystoscopy, for example, will be new equipment and procedures for them) and should be aware of safety features, warning mechanisms, and alarms on the device [2]. For example, it has been shown that serious complications occurred with use of global endometrial ablation devices when there was nonadherence to manufacturer’s protocols or when the safety features of the devices were “over-ridden” [4]. Nurses and medical assistants should read and periodically review manufacturer’s instructions and have them available for all team members should questions about the procedure arise.
Sterilization
In terms of sterilization, the initial decision is to determine if this is to be done in the office itself or sent to an outside vendor for processing and sterilization. If the process is to be done by an outside vendor, the surgeon and staff need to be aware of scheduling and stocking of equipment in order to ensure needed equipment such as the cystoscope or hysteroscope is clean and available. If this is done in the office, who will be completing this, and how will they be kept in compliance for maintaining the instruments? Some institutions have specific protocols that are followed in regards to sterilization (see policy for your specific institution) but stand-alone offices will need to have one.
The ultimate goal of disinfection and sterilization in office surgical practices is to reduce rates of health care-associated infections through appropriate use of both disinfection and sterilization [5]. Therefore, the type of sterilization should be addressed first. Once this has been decided, keep in mind that is important to always clean first, then disinfect or sterilize [6]. This includes removing visible tissue and fluids to allow for the removal of microorganisms and organic matter. Shortly after use, take apart the equipment for cleaning and reassemble it on the field. Sterilize all moving parts in the open position to ensure proper cleaning. If there is a question about how an instrument should be cleaned, it is suggested to first follow the manufacturer’s guidelines to avoid damaging an instrument or shortening its usefulness due to unnecessary wear and tear from improper maintenance.
All endoscopes should be disconnected and disassembled and cleaned with an enzymatic cleaner that is suitable with the cystoscope or hysteroscope (Fig. 2.2). Cleaning is needed before disinfection or sterilization. Also, single piece, reusable medical equipment such as single tooth tenaculum, Allis clamp or uterine sound should also be sterilized: each office will need to decide if it will sterilize each piece individually or as a procedure “set” and follow its own policy. Also, quality control and monitoring of sterilization equipment and of infection control is recommended and should be monitored and reviewed by the Medical Director (see below). Lastly, local and state standards and policies should be reviewed relative to the office location as well as compliance with Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and Occupational Safety and Health Administration (OSHA) requirements.
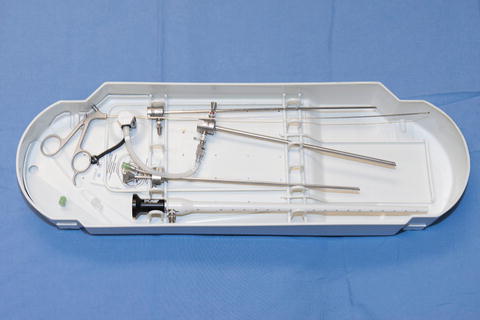

Fig. 2.2
Example of disassembly of an office hysterocope prior to sterilization
If sterilization will be done in the office there are several types of sterilization techniques to consider, and the choice should be determined by what is most readily available and appropriate for each office. Time, space, and cost are factors to determine when considering set-up of sterilization system. Also, the office should be certain to utilize a Food and Drug Administration (FDA)-cleared liquid chemical sterilant and high-level disinfectant that can be used to reprocess office endoscopes and other equipment [5]. Below is a brief summary of common sterilization processes as outlined by Bradley and Fluharty [6].
Steam Sterilization
Steam is the preferred method for sterilizing critical medical and surgical instruments that are not damaged by heat, steam pressure, or moisture (CDC). Sterilization using steam is hazardous to telescopes and light cables unless they are wrapped for prevacuum sterilization or are flash sterilized when they are unwrapped. The prevacuum step takes about 45 min and is a multistep process. The flash sterilization is performed at high temperatures and high pressures. A drying phase may be added. Equipment must then be used immediately after sterilization. The equipment must be handled carefully because it is very hot.
Steris
Steris systems are becoming more popular due to the ability to rapidly disinfect equipment. Instruments are immersed in the buffered solution of 35 % peracetic acid and then treated in a high-agitation system. The time from start to finish is around 30 min. Instruments need to be used immediately after sterilization. The Steris system works by a combination of hydrogen peroxide combined with a low-temperature gas plasma system that produces low temperatures (50–104 °F).
Ethylene Oxide Gas Sterilization
Sterilization with ethylene oxide takes about 12 h to complete. Instruments must be totally dry when employing this method. All instruments must be properly aerated to remove residual toxic gas. Many health care facilities are starting to require other methods of sterilization as this method is restricted in some communities.
Single-use equipment, such as catheters for urodynamics, hysteroscopic sterilization catheters (microcoils), and loops for electo-excision procedures, should be used once and then disposed of in appropriate hazardous waste containers. If transvaginal ultrasound equipment is used with saline infusion sonography (SIS), appropriate cleaning and disinfection of the transvaginal probe is needed. Disposable single-use endovaginal probe covers have significant leakage rates and as such, the probe should undergo high-level disinfection of the probe between each use even if the probe cover appears intact [7].
Finally, standard office cleaning and disinfection should be undertaken after each office-based procedure to other equipment such as colposcopes (wiping down handles and dials), urodynamics equipment such as the urochair and other procedural office equipment.
Anesthesia and Analgesia
The next area to be addressed in regards to office set-up should be that of anesthesia and analgesia. This must be dictated by the type of procedure to be performed as well as input from the patient. The type of anesthesia used should never be altered due to limitations of equipment or personnel in the office setting. If the appropriate anesthesia needed for a specific case or a specific patient cannot be accommodated, then the case should be done in a more acute facility so it can be done safely and efficiently. With this in mind, the level of anesthesia (which includes light, moderate, or deep sedation) will dictate the equipment and the appropriate personnel needed for the case.
Once a procedure is scheduled in the office, it is necessary to first decide which level of anesthesia the office will provide, as the level of sedation needed for the procedure dictates specific protocols as listed below. The American Society of Anesthesiologists (ASA) has developed guidelines for sedation and these are discussed in more detail in Chap. 4. Level one includes the use of local anesthesia with minimal preoperative use of oral anxiolytics. Level two is moderate sedation, and level three is considered deep sedation or general anesthesia. A majority of the procedures in this text will likely need only local anesthetic with level-one sedation. On some occasions, level-two sedation may be needed. It is recommended by these authors that if sedation beyond level 2 is needed, consideration of assistance in office from an anesthesia related provider (Certified Registered Nurse Anesthetist (CRNA) or anesthiesiologist) be available during the procedure or consideration of performance of the procedure in an ambulatory surgery center (ASC) or monitored facility. The ability to rescue a patient from sedation is based on the level of anesthesia being used. Each office should develop a policy for emergency medication, resuscitation, and have the ability to rescue a patient from excessive medication. In the Report of the Presidential Task Force on Patient Safety in the Office Setting [5], it is recommended that these policies be based on ASA levels depending on the level of invasiveness.
Specifics for both local anesthetics and use of adjunct medications of analgesia or sedation are discussed more fully in Chap. 4. However, it is also advisable that gynecologic surgeons planning to perform in-office procedures review the document, “Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists” [8].
Use of Medications
In-office anesthesia and analgesia requires assessment of in-office medication use and safety. All medications necessary to complete a specific procedure need to be present and immediately available in the procedure room for use during an in-office surgery; this includes mediations that may be necessary to reverse or “rescue” any potential complications or oversedation. When a procedure will include a controlled medication, this must be stored, registered, and removed from a secure location. All medication use needs to be recorded and monitored with a medication log including the use of local anesthetics as well as controlled substances such as anxiolytics and medications used for pain relief. Medications need to be periodically checked for expiration dates to allow replacement as needed. It is frustrating to schedule an in-office surgery only to find that an important medication needed has passed its expiration date. The person(s) responsible for the medications needs to be present in the room during the procedure. Who this person is depends on the level of anesthesia that is being used. The responsible party ranges from nurse, CRNA, or anesthesiologist. The surgeon assumes responsibility for the first two. Once the procedure is complete, patient monitoring postoperatively will need to be correlated to the level of sedation achieved during the surgery. Many states are now requiring specific accreditation dependent upon if moderate sedation or deeper anesthesia is to be administered in office [9] and most states have regulations set forth by state pharmacy boards for specifics regarding use and distribution of in-office medication. Each physician is advised to avail him or herself of state and local guidelines regarding purchase, use, and storage of medications in the office setting.
Medication safety has been clearly recognized as a source of patient harm in the office setting [10]. One first step to overcome this potential problem is a clear process to assess patients’ medications and allergies before beginning the procedure. While the advent of the computerized medical record has seemingly streamlined this process, a member of the office team should document medications and allergies prior to the start of the procedure as well as inform the surgeon and members of the team at the beginning of the procedure, such as during a procedural “time out”. Use of standardized doses and schedules for each procedure are recommended. However, during or after the procedure and during urgent situations in the office, increased stress or the use of “verbal orders” may increase the possibility of error in prescribing and administering or monitoring medications [11].
Joint Commission has published Ambulatory Care National Safety Goals (NPSG) with specifics to medication safety in the ambulatory setting. In regards to medication safety, NPSG.03.04.01 Improve the safety of giving medications states,” Label all medications, medication containers, and other solutions on and off the sterile field in perioperative and other procedural settings. Note that medication containers include syringes, medicine cups, and basins” [12]. This means that any medication, whether given orally, intravenously (IV), or intramuscularly (IM) to the patient before the procedure, used in a syringe during a procedure (such as with local anesthesia) or if given in oral, IV or IM in recovery after the procedure should have labels that include the following:
Medication name
Strength
Quantity
Diluent and volume (if not apparent from the container)
Expiration date when not used within 24 h
Expiration time when expiration occurs in less than 24 h
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


