Fig. 20.1
Oblique sonogram of a 16-week gestational age fetus demonstrates a 1 cm teratoma emanating from the right cheek into the amniotic fluid of an otherwise healthy 30-year-old gravida 4, para 1 woman
The development of fetal MRI has allowed for the imaging of fetal masses with an exceptional degree of detail (Fig. 20.2) . This degree of detail is essential for determining the appropriate management of large teratomas of the head and neck, particularly in regard to whether an EXIT procedure is indicated and to establish what should be done to secure the airway and manage the tumor during and after the EXIT is performed . T2-weighted MRI images in a normal fetus should demonstrate a hyperintense signal throughout the tracheal column, indicating a patent, fluid-filled airway. The absence of such a signal suggests complete high airway obstruction syndrome (CHAOS) as a result of the lesion, indicating that tracheostomy in conjunction with an EXIT may be required. If a tracheostomy is determined to be necessary, then the path of a severely deviated trachea may be predicted from the fetal MRI images [48].
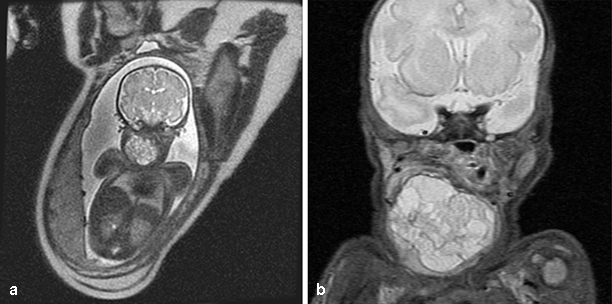
Fig. 20.2
a Prenatal MRI of a patient obtained at 34 weeks gestational age showing a large heterogeneous mass emanating from the right neck. A bronchoscopy and intubation were performed in conjunction with an EXIT procedure. b A postnatal MRI of the same patient obtained on the first day of life showing similar findings. The mass was resected 2 weeks later
Though there is no evidence of risks to the fetus from MRI, the safety of MRI in pregnancy has not yet been clearly defined. Thus, the Safety Committee of the Society of MRI has deemed fetal MRI to be appropriate only in cases where other imaging methods, such as ultrasound, are determined to be inadequate or when the MRI will provide important additional information [50, 51].
MRI is helpful in delineating the tumor prior to surgery. In malignant GCT of the head and neck in infants and older children, MRI has a characteristic appearance with uniform signal intensity within a well-defined mass, hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging [43]. This differentiates these tumors from vascular lesions such as LM which have a multilocular appearance and poorly defined margins, and from rhabdomyosarcomas which infiltrate surrounding structures.
The role of CT in prenatal imaging is greatly limited, due to the risk of radiation exposure to the fetus and limited soft-tissue detail in comparison to MRI. Its prenatal use has been reported where a high suspicion for progressive bony destruction of the face exists, allowing for determination of the need for early delivery and resection to prevent further facial destruction [52]. CT does play a role in identifying invasion of bone when planning for the resection of lesions diagnosed in the postnatal period that are adjacent to the skull base, palate, or facial bones.
PET scanning is not routinely used in the evaluation of pediatric GCT, but malignant GCT of head and neck are PET-avid and extrapolating from experience with adult nongerminomatous GCT; this modality may have an increasing role to play in monitoring response to therapy [53].
Pathology
Grossly, teratomas typically have a multinodular outer appearance with a fleshy whitish yellow interior with dark brown areas and foci of hemorrhage (Fig. 20.3). Microscopically, a teratoma is a true congenital neoplasm, and, by definition, contains tissues originating from each of the three major germ cell layers, namely ectoderm, mesoderm, and endoderm (Fig. 20.4) . Approximately 68 % of cervical teratomas also contain neuroectodermal elements, such as neural tissue [25]. These tissues present in a variety of degrees of differentiation, ranging from immature cells to highly developed tissue structures, such as pancreas and intestine. In children and adults, tumors with an increased proportion of immature elements often have elevated levels of AFP and are more prone to recurrence. Teratoid cysts are poorly differentiated tumors developed from all three germ layers, and an epignathus is an intraoral teratoma originating from the base of the skull that may have completely developed fetal organs or limbs [54]. Cervical teratomas also frequently contain thyroid tissue, though their relationship to the thyroid gland itself varies.
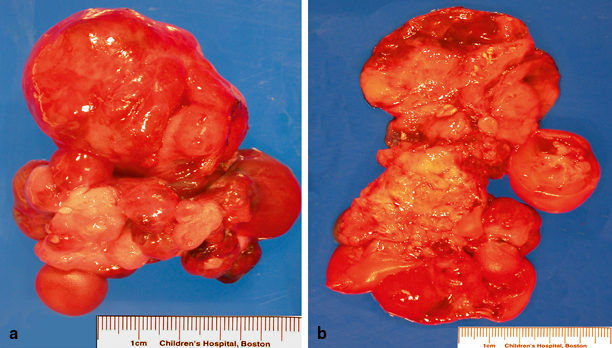
Fig. 20.3
Macroscopy of palatal solid immature teratoma. a Large soft-tissue mass with red brown smooth surface and a multinodular appearance. b On cut surface, the mass is fleshy with whitish yellow and dark brown areas as well as foci of hemorrhage
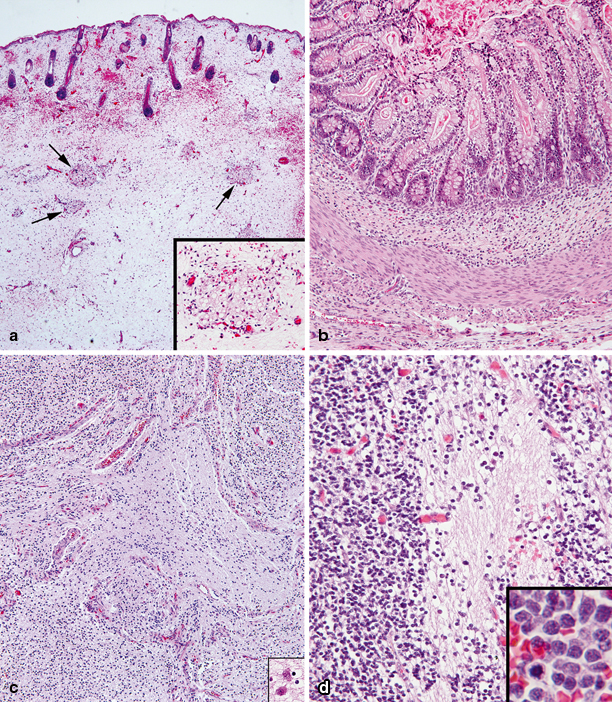
Fig. 20.4
Light microscopy of palatal solid immature teratoma. a Ectodermal and mesodermal components with developing skin (on top) and immature subcutaneous tissue with islands of developing adipose tissue (arrows and inset). b Endodermal and mesodermal components with well-formed intestinal structure including intestinal mucosa, muscularis mucosae, submucosa, and muscularis propria. c Neurectodermal component with disorganized developing brain tissue. d Poorly differentiated neuroblastic elements with abundant neuropil (center). Neuroblasts at higher magnification are seen in the inset; occasional cells are undergoing mitoses
Histopathologically, malignant GCT of the head and neck with a component of endodermal sinus tumor were first described by Teilum in 1959, and are typically characterized by Schiller-Duval bodies that consist of a single row of tumor cells surrounding a blood vessel that is in turn surrounded by a cystic space (Fig. 20.5) [1]. Immunohistochemical tests find that the cytoplasm of the tumor cells usually stains strongly positive for AFP and Lin28. Cytogenetic changes in chromosome 1, 3, and 6 may be found, but, unlike adult yolk sac tumors, isochromosome 12p is rarely seen [55].
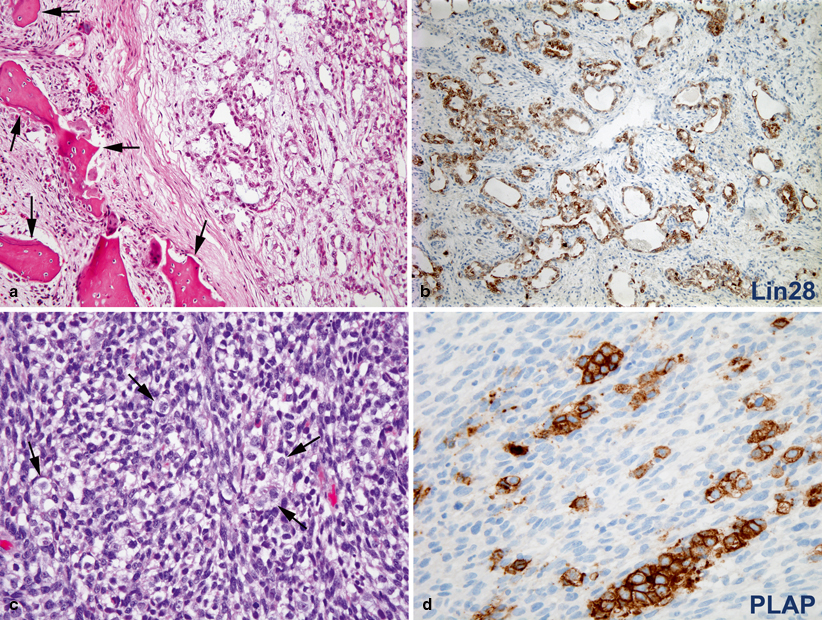
Fig. 20.5
a Endodermal sinus tumor (yolk sac tumor) arising within the maxillary bone (arrows). The tumor (right) is composed of irregular anastomosing glands in a loose myxoid stroma. b Tumor cells show diffuse and strong immunoreactivity for Lin28, a highly sensitive and useful marker for the diagnosis of these tumors. c Sarcomatous component of a germ cell tumor primary of the central nervous system. The tumor is predominantly composed of poorly differentiated spindled cells. Scattered large round dysgerminoma cells with clear cytoplasm are indicated by arrows. d The cytoplasm of dysgerminoma cells is strongly immunoreactive for placental alkaline phosphatase
Treatment
Medical
Despite their benign histology, teratomas of the head and neck region constitute a life-threatening and potentially fatal disease. The main intervention is surgical, and modern surgical techniques, accompanied by innovative supportive care, have resulted in survival rates in excess of 80 %.
During infancy, the risk of a GCT being malignant increases with age, and GCT of the head and neck in children in the first decade of life that are older than 1 year of age are primarily likely to be malignant. Due to concern about a biological switch from histologically benign teratomas in the perinatal period to mixed malignant GCT in older children, complete resection of these tumors is considered crucial [56].
A MEDLINE review revealed 45 cases of malignant GCT of the head and neck in children, published in a variety of case reports and case series (Table 20.1). Complete surgical resection is considered a keystone to cure [57]. Earlier chemotherapy regimens that included vincristine, actinomycin D, and cyclophosphamide improved survival; however, it was the introduction of Einhorn’s cis-platinum-based therapy, which included etoposide and bleomycin, that produced significant improvement in disease-free survival rates in children with malignant GCT [2, 3, 58]. Given the rarity of pediatric GCT, recent progress in treating patients has occurred largely as a result of several multi-institutional trials around the world. In North America, this includes the collaborative efforts of the Children’s Oncology Group (COG), the merged entity of the former Children’s Cancer Group (CCG) and the Pediatric Oncology Group (POG) [59]. For patients with head and neck malignant GCT who recur prior to or after surgery, the choice of salvage therapy includes TIP (i.e., paclitaxel (Taxol®) 250 mg/m2 + ifosfamide 1.2 g/m2 × 5 + cisplatin 20 mg/m2 × 5), high-dose chemotherapy (HD-CT) and autologous stem cell transplant (ASCT), and TIC (i.e., paclitaxel (Taxol®) (135 mg/m2 × 1), ifosfamide (3 g/m2 × 5) and carboplatin (560 mg/m2 × 1)) [60].
Table 20.1
Outcome for pediatric patients with extracranial malignant GCT of the head and neck
Age | Sex | Location | Surgery | RT | Chemotherapy | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
NB | F | OP | PR | Yes | Actinomycin D, 5FU, thio-TEPA | DOD 15 m | [28] |
NB | ND | Hypopharynx, neck | Resection | No | VAC × 3 cycles | NED 5 y | [2] |
NB | ND | Hypopharynx, neck | Resection | No | VAC × 3 cycles | NED 5 y | [2] |
NB | M | Anterior neck | Resection | No | None | NED 13 y | [26] |
6 w | F | Forehead | PR | 52 Gy | Actinomycin D, PVB, CTX | DOD 4.5 m | [26] |
3 m | ND | Orbit | Biopsy | No | Chemo NOS | ND | [42] |
4 m | F | Mandible | Resection | No | PEB × 4 cycles, TIP × 4 cycles | NED 5 y | [32] |
4.5 m | F | Face | Resection | No | None | NED 10.5 m | [26] |
6 m | F | Floor of mouth | PR | Yes | None | DOD, 7 m | [28] |
6 m | F | Orbit, maxilla, PS | PR | No | None | DOD 5 m | [39] |
6 m | M | Orbit, nasal cavity, PS | Biopsy | No | PEB × 4 cycles | NED 6 m | [44] |
7 m | F | OP | PR | No | None | DOD 17 d | [27] |
8 m | F | Ear | Resection | No | PEB | NED 13 m | [61] |
9 m | F | Masticator space | None | No | PEB × 4 cycles | ND | [30] |
10 m | F | PS | Biopsy | No | None | DOD 1 m | [31] |
10 m | F | Nasopharynx | Biopsy | Yes | Chemo NOS | DOD 9 m | [28] |
12 m | F | Neck | Biopsy | No | PVB | DOI | [39] |
12 m | M | Orbit | PR | No | PEB × 3 cycles | NED 4 m | [45] |
13 m | F | Orbit | Exenteration | No | VAC | DOD 10 m | [42] |
13 m | F | Orbit | None | Yes | None | NED 8 m | [42] |
13 m | ND | Hypopharynx, neck | Resection | No | PVB/EI | NED 5 y | [2] |
15 m | M | Orbit, nasopharynx | None | 60 Gy | C5V × 12 cycles, VCR, CTX, 5FU | NED 8.5 y | [41] |
18 m | ND | Hypopharynx, neck | Resection | No | PVB/EI | NED 5 y | [2] |
18 m | F | Ear | Biopsy | No | VAC/PVB | NED 15 m | [36] |
18 m | F | External auditory canal | Biopsy | No | PEB × 10 cycles | NED 36 m | [62] |
18 m | M | Palate, alveolus, OP | Biopsy | No | VAC | ND | [39] |
18 m | M | Orbit | Exenteration | No | C5V | NED 8 y | [42] |
19 m | F | Submandibular region | Biopsy | Yes | Chemo NOS | DOD 5 m | [38] |
20 m | F | Temporal area | PR | No | PEB × 6 cycles | NED 5 m | [63] |
23 m | F | Temporomandibular | PR | No | Chemo NOS | NED 40 m | [64] |
24 m | F | Parotid space | Resection | Yes | Chemo NOS | DOD 18 m | [33] |
24 m | M | Nasal cavity | Resection | Yes | Chemo NOS | NED 4y | [65] |
26 m | F | Ear | Resection | No | None | DOD 3 m | [66] |
30 m | F | Postauricular region | None | Yes | Chemo NOS | NED 3 y | [26] |
30 m | F | Orbit | PR | No | PEB × 6 cycles | NED 9 y | [67] |
33 m | ND
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|