Fig. 8.1
Hypothetical mechanism of gene and environment interactions in the development of obsessive–compulsive symptoms in schizophrenia. OCSs obsessive–compulsive symptoms, OCD obsessive–compulsive disorder, SCZ schizophrenia, E environment, G gene
On the other hand, as both schizophrenia and OCD are expected to have a heterogeneous genetic basis with a large number of susceptibility genes, some degree of their co-occurrence might be explained by the presence of common genes with a pleiotropic effect that have not been identified so far. Also, considering the phenomenological similarity between obsession and delusion (Bottas et al. 2005) (for details on the similarities and differences between delusion and obsession, see Chap. 3), OCSs occurring only in the context of psychosis might be a special manifestation of the psychotic processes that are not related to the vulnerability to OCD.
Considering that most of the specific genetic and environmental factors in schizophrenia and OCD have not been identified as yet and that the biological mechanism of gene–environment interactions in psychiatric illness is not well established, testing the abovementioned hypotheses in the field of neurobehavioral genetics requires many prerequisite steps. According to a recent meta-analysis of twin studies (Taylor 2011) on OCD, additive genetic factors explained ~37–41 % of phenotypic variance, and non-shared (individual-specific) environment accounted for ~50–52 % of the variance. Several environmental factors including perinatal events, infections, traumatic life events, and stressors have been suggested to trigger OCSs (Geller et al. 2008; Grisham et al. 2011; Lafleur et al. 2011; Landau et al. 2011; Murphy et al. 2010). Compared to these factors, SGAs would be an environmental factor that is less complicated and can be reliably measured. Therefore, genetic studies on the SGA-induced OCSs could provide an important insight into the gene–environment interaction mechanism. For genetic studies of OCSs in schizophrenia, detailed characterization and subgrouping of OCSs in schizophrenia is also required. Until now, only a small number of studies have generated genetic data regarding OCSs in schizophrenia. These were mostly candidate gene association studies, and the phenotype was OCSs in general or SGA-induced OCSs manifested in schizophrenia. Although these are mostly unreplicated studies with a small sample size, these studies would be an important initial step for exploring the complete genetic architecture of OCSs in schizophrenia.
8.2 Genetic Studies on Obsessive–Compulsive Symptoms in Schizophrenia
Studies in twin pairs with schizophrenia found greater resemblance for symptomatic profile in monozygotic twins than in dizygotic twins, which suggests the influence of genetic factors on clinical features of schizophrenia (Cardno et al. 2001, 2002). Some family studies reported that various factorial dimensions of schizophrenia showed significant within-family correlations (Wickham et al. 2001; Choi et al. 2007; Vassos et al. 2008). However, in contrast to vigorous exploration of disease susceptibility genes, genetic approaches for exploring the genes related to specific symptoms or sub-phenotypes of schizophrenia are very limited. Until now, the most commonly investigated symptom dimensions in genetic linkage or association studies are reality distortion (positive), psychomotor poverty (negative), disorganization, and manic or depressive dimensions (Fanous et al. 2007, 2012; Hamshere et al. 2011). OCS or comorbid OCD has seldom been measured in large-scale genetic studies exploring modifier genes for comprehensive clinical symptom dimensions of schizophrenia.
In a recent genome-wide quantitative trait locus (QTL) linkage analysis, our group (Ryu et al. 2013) assessed clinical symptoms of a total of 315 schizophrenia patients on a lifetime basis. From 42 indicators measured using the Korean version of the Diagnostic Interview for Genetic Studies (DIGS) (Joo et al. 2004), eight quantitative phenotypes representing symptom dimensions were identified through the principal component factor analysis, i.e., “auditory hallucination factor,” “Schneiderian first-rank symptom factor,” “paranoid factor,” “non-paranoid delusion factor,” “somatic preoccupation factor,” “prodromal impairment factor,” “negative symptom factor,” and “disorganization factor.” In this study, lifetime OCSs were loaded on the “somatic preoccupation factor” together with tactile, gustatory, and olfactory hallucinations and somatic delusions. The loading value of OCSs on this dimension was 0.47, which was lower than that of tactile hallucination (0.69) and somatic delusion (0.60) and comparable to that of gustatory hallucination (0.49) and olfactory hallucination (0.45). This phenotype dimension could not be genetically validated in our QTL linkage analyses, which showed no significant linkage signal for this dimension (Ryu et al. 2013). In a recent clinical analysis of schizophrenia patients, our group observed that this symptom dimension was associated with earlier age of onset (unpublished data). Further validation of this dimension as a unique sub-phenotype of schizophrenia is warranted. In future studies, heterogeneity of the OCSs manifested in the lifetime course of schizophrenia needs to be considered.
In selecting candidate genes for association studies on schizophrenia with OCD or OCSs, previous findings of genetic studies for OCD could be considered. The results of GWASs and meta-analyses of candidate gene studies suggest that the genes involved in the glutamatergic, serotonergic, and dopaminergic systems and neurotrophic factors may contribute to the risk of OCD (Pauls et al. 2014). There were genetic association studies specifically focusing on schizophrenia with OCD. The catechol-O-methyltransferase (COMT) gene was the first candidate gene tested due to its crucial role in dopaminergic neurotransmission and prefrontal cognitive functions (Denys et al. 2004). Poyurovsky and colleagues (Poyurovsky et al. 2005b) investigated the association of Val158Met polymorphism of the COMT gene with comorbid OCD in schizophrenia in the Israeli population and reported negative results. In a subsequent study, Zinkstok and colleagues (Zinkstok et al. 2008) observed a significant effect of the COMT genotype on the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) (Goodman et al. 1989) scores in male patients with recent-onset schizophrenia in the Netherlands. The Val/Val genotype of COMT Val158Met polymorphism, the genotype of high activity, was associated with high Y-BOCS scores. The authors suggested that the COMT gene may be a modifier gene for the symptomatology of schizophrenia.
The brain-derived neurotrophic factor (BDNF) gene was another candidate gene for OCSs in schizophrenia. It is involved in the brain development and neurotransmitter systems known to be implicated in schizophrenia and OCD (Wang et al. 2011; Favalli et al. 2012). In a recent study by Hashim et al. (2012), the BDNF Val66Met polymorphism was significantly associated with OCSs in the Egyptian patients with schizophrenia. The Val allele was significantly higher in the with-OCS group compared to the without-OCS group. The mean Y-BOCS scores were significantly different among the three genotype groups: Val/Val > Val/Met > Met/Met.
Until now, genetic studies on OCSs in schizophrenia provided limited information due to a small sample size and few replication trials. However, these studies have shown the possibility of OCSs being a meaningful phenotype in the exploration of the genetic basis of course and symptom modifications in schizophrenia. Many more candidate genes related to the biological mechanism of schizophrenia and/or OCD need to be tested. Larger-scale linkage analysis and GWAS for this phenotype are also warranted. To construct a reliable basis of replication and meta-analyses, a more standardized definition for the phenotype should be applied in future studies.
8.3 Antipsychotic-Induced Obsessive–Compulsive Symptoms as a Phenotype
Since the introduction of clozapine and other SGAs, case reports (Lykouras et al. 2003) and clinical studies (Baker et al. 1992; de Haan et al. 1999; Ertugrul et al. 2005; Mahendran et al. 2007; Scheltema Beduin et al. 2012) have described the de novo onset of OCSs during treatment with these drugs (for details see Chap. 10). Our group previously screened 209 clinically stable schizophrenia outpatients receiving SGAs and detected OCSs in 44 patients (21.1 %) (Lim et al. 2007). OCSs associated with SGAs were identified in 26 patients (12.4 % of total patients and 59.1 % of patients with OCSs) (Lim et al. 2007). In this study, patient diagnosis was performed through a direct interview using the Korean versions of the Structured Clinical Interview Schedule for DSM-IV Axis I Disorders (K-SCID-I) (Hahn et al. 2000) and the Y-BOCS (Kim et al. 2004). We proposed the following criteria for SGA-induced OCSs: (1) meet all of the criteria of DSM-IV OCD except for criteria C (descriptions of severity) and E (exclusion of drug-induced cases); (2) OCSs are not the direct result of delusions, and their contents are clearly different from their residual psychotic symptoms; and (3) OCSs were developed de novo while receiving SGAs. Patients with a history of transient OCSs in childhood or adolescence were considered to have de novo OCSs if they experienced substantial OCS-free periods before the onset of psychotic illness. Although only 35.9 % of the study cohort was taking clozapine at the time of the evaluation, 76.9 % of SGA-induced OCSs were associated with clozapine treatment.
Among the various subgroups of comorbid OCSs in schizophrenia, SGA-induced OCSs might be more homogeneous in terms of biological mechanisms. It could also be a valid phenotype for pharmacogenetic studies aiming at individualized medicine. Since the SGA-induced OCSs are delayed adverse effects that cause great distress to the patients (Lim et al. 2007), and there are differences in the OCS-inducing propensity among SGAs, i.e., high in clozapine and olanzapine and low in amisulpride and aripiprazole (Schirmbeck et al. 2013), evaluation of an individual’s susceptibility to SGA-induced OCSs in the early stage of medication would be highly helpful in the pharmacotherapy of schizophrenia.
It is widely accepted that genetic factors play an important role in an individual’s response to specific drugs (Malhotra 2002; Arranz and de Leon 2007). However, heritability data for drug responses are extremely limited. Our group previously described clozapine-induced OCSs that emerged concordantly in a pair of monozygotic twins (Hong et al. 2008). More case reports and data from clinical surveys need to be accumulated to clarify the role of genetic factors. Another indirect evidence of genetic control of SGA-induced OCSs is the strong heritability of OCD reported in family and twin studies (Hanna et al. 2005; van Grootheest et al. 2005; Mataix-Cols et al. 2013; Pauls et al. 2014).
If there is a genetic vulnerability to SGA-induced OCSs, would it be related to the vulnerability to OCD? An indirect approach to find an answer to this question would be analyses of the phenomenological similarity between SGA-induced OCSs and OCD. For determining whether SGA-induced OCSs have the same symptom characteristics as those displayed in OCD, our group evaluated the frequencies and dimensions of SGA-induced OCSs measured using the Y-BOCS (Kim et al. 2004) in schizophrenia patients (Kim et al. 2012). Frequencies of 13 symptom categories were very similar to those in Korean OCD patients (Ha et al. 2004), showing statistically significant intraclass correlation (ICC = 0.77, P < 0.01) (Fig. 8.2, adopted from the study by Kim et al. 2012). The factor structure yielded from the principal component analysis was to a large extent comparable not only to that demonstrated in the previous study among Korean OCD patients (Ha et al. 2004) (Table 8.1, adopted from Kim et al. 2012) but also to that reported in other studies of OCD patients (Leckman et al. 1997; Hasler et al. 2005, 2007; Bloch et al. 2008), suggesting that a common biological mechanism underlies the two clinical conditions. In contrast, in a previous study of schizophrenia patients with comorbid OCD (Faragian et al. 2009) that excluded patients with drug-induced OCSs from their study, a somewhat different symptom structure was generated. In that study, only the cleaning factor was comparable to that in previous studies of pure OCD. Other factors were constructed by a combination of symptoms from different dimensions in previous OCD studies (Leckman et al. 1997; Hasler et al. 2005, 2007; Bloch et al. 2008).
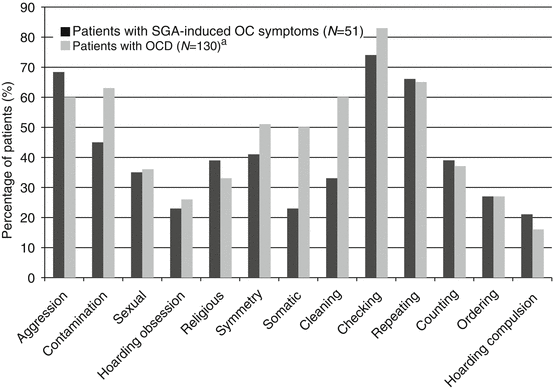

Fig. 8.2
Symptom frequencies for 13 categories of the Y-BOCS symptom checklist in patients with SGA-induced obsessive–compulsive symptoms and patients with OCD. Each category of the Y-BOCS symptom checklist was scored as 0 = absent or 1 = present. The symptom frequency (y-axis) was defined as the percentage of patients scored as “1” in the category. Symptom frequencies showed a significant correlation between two groups (ICC = 0.77, P < 0.001) (Reproduced from Kim et al. 2012). Y-BOCS yale–brown obsessive–compulsive scale, SGA second-generation antipsychotics, OCD obsessive–compulsive disorder, ICC intraclass correlation coefficient. aHa et al. (2004)
Table 8.1
Comparison of factor structures and loadings for the Yale–Brown Obsessive–Compulsive Scale symptom categories between schizophrenia patients with second-generation antipsychotic-induced obsessive–compulsive symptoms and patients with obsessive–compulsive disorder
Symptom category | SGA-induced OC symptoms (N = 51) | Obsessive–compulsive disorder (Ha et al. 2004 a) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Forbidden thoughts | Hoarding | Cleaning | Symmetry | Counting | Contamination/cleaning | Symmetry/ordering | Pure obsessions | Hoarding | Repeating/counting | |
Aggressive | 0.80 | 0.33 | 0.01 | −0.06 | 0.19 | −0.19 | −0.19 | 0.71 | 0.10 | −0.10 |
Contamination | 0.37 | −0.12 | 0.80 | −0.13 | −0.03 | 0.92 | −0.05 | 0.05 | 0.05 | −0.04 |
Sexual | 0.62 | 0.05 | 0.12 | 0.16 | −0.54 | 0.15 | −0.07 | 0.78 | −0.01 | 0.00 |
Hoardingb | 0.18 | 0.85 | −0.08 | −0.07 | −0.01 | 0.08 | 0.12 | 0.04 | 0.86 | 0.10 |
Religious | 0.55 | 0.37 | 0.15 | 0.24 | −0.23 | 0.32 | 0.11 | 0.51 | −0.04 | 0.15 |
Symmetry | 0.24 | −0.16 | 0.14 | 0.76 | 0.02 | 0.01 | 0.80 | 0.03 | 0.10 | 0.23 |
Somatic | 0.60 | −0.33 | −0.02 | 0.26 | −0.07 | 0.01 | 0.38 | 0.34 | 0.19 | −0.13 |
Cleaning | 0.02 | 0.22 | 0.84 | 0.18 | 0.15 | 0.89 | 0.11 | −0.01 | 0.09 | −0.03 |
Checking | 0.74 | 0.28 | 0.08 | −0.00 | 0.16 | −0.20 | 0.39 | 0.49 | 0.03 | 0.00 |
Repeating | 0.23 | 0.20 | −0.29 | 0.59 | 0.04 | −0.06 | 0.24 | 0.04 | 0.10 | 0.79 |
Counting | 0.19 | 0.04 | 0.16 | 0.10 | 0.83 | 0.02 | −0.02 | −0.06 | 0.15 | 0.83 |
Ordering | −0.27 | 0.22 | 0.48 | 0.66 | −0.01 | 0.12 | 0.84 | −0.17 | 0.02 | 0.07 |
Hoardingc | 0.21 | 0.80 | 0.27 | 0.20 | 0.04 | 0.05 | 0.05 | 0.04 | 0.86 | 0.16 |
Percent variance (%) | 19.35 | 15.81 | 14.21 | 12.59 | 8.80 | 14.41 | 13.71 | 13.66 | 12.20 | 11.27 |
Pharmacogenetic studies would not only be important for the individualized treatment but also for uncovering the biological mechanism of SGA-induced OCSs. To promote the progress of these studies, more clinical concerns regarding this phenotype and more heritability data including assessment of concordance between twin pairs or relative pairs are required. Analyses of phenomenological similarities between SGA-induced OCSs and OCD or other subgroups of OCSs in schizophrenia are also helpful to clearly determine the boundary of the phenotype.
8.4 Pharmacogenetic Studies on Antipsychotic-Induced Obsessive–Compulsive Symptoms
To date, several pharmacogenetic studies on SGA-induced OCSs have been performed. Owing to the short history of genetic researches on this topic and difficulties in large-scale patient recruitment, these are all candidate gene association studies with a small sample size. Genes coding for proteins involved in both the pharmacodynamic actions of SGAs and neurobiological processes of OCD, i.e., genes related to serotonergic, dopaminergic, and glutamatergic neurotransmission (Lopez-Gil et al. 2010), could be reasonable candidate genes. A few genes involved in the glutamatergic system have shown positive association with SGA-induced OCSs.
Our group investigated the glutamate transporter gene, SLC1A1, that was regarded as one of the most promising candidate genes for OCD (Arnold et al. 2006; Dickel et al. 2006; Stewart et al. 2007). Subjects included 94 clinically stable Korean patients with schizophrenia under SGA treatment. The obsessive–compulsive (OC) group was defined based on the criteria proposed in our previous study (Lim et al. 2007) (described in the previous section), and the non-OC group consisted of patients who had received SGA for more than 24 months and did not develop OCSs. Ten SNPs of SLC1A1 were selected based on the previous reports on OCD, and the genotype- and haplotype-based association was tested using a logistic regression model considering age, sex, and medication type as covariates. We observed trends of association in rs2228622 and rs3780412 (nominal P < 0.05, adjusted permutation P < 0.1) for the dominant model that was the best inheritance model fitting our data. In the haplotype-based analysis, the A/C/G haplotype at rs2228622-rs3780413-rs3780412 showed a significant association with SGA-induced OCSs, and this association withstood multiple test correction (nominal P = 0.011, adjusted permutation P = 0.038, OR = 3.955, 95 % CI 1.366–11.452, for dominant model) (Fig. 8.3). The same nature of association was observed in the separate analysis of the subgroup of patients receiving clozapine treatment. This was the first study that provided preliminary evidence of the involvement of glutamatergic neurotransmission in the pathogenesis of SGA-induced OCSs.
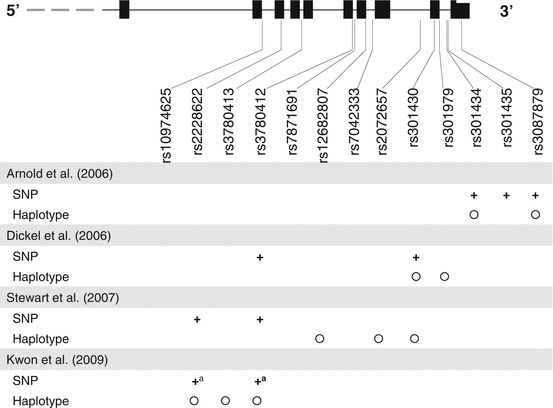

Fig. 8.3
SLC1A1 organization and single-nucleotide polymorphism (SNP) locations (from the University of California at Santa Cruz Genome Browser, Mar 2006 Assembly). The horizontal line represents the genomic sequence, and vertical bars represent exons. Plus signs and circles denote SNPs significantly associated with obsessive–compulsive disorder (Arnold et al. 2006; Dickel et al. 2006; Stewart et al. 2007) or second-generation antipsychotic-induced obsessive–compulsive symptoms (Kwon et al. 2009) in single-marker and haplotype analyses. aOnly trends of association were observed with nominal P < 0.05
In a succeeding investigation, our group tested another candidate gene involved in the glutamatergic neurotransmission, the discs, large-associated protein 3 gene (DLGAP3), also known as SAP90/PSD-95-associated protein 3 gene (SAPAP3) (Ryu et al. 2011). In that study, we also explored the interaction effect of DLGAP3 and SLC1A1 on SGA-induced OCSs. Among the seven tag SNPs of DLGAP3, the only nominally significant association was found in rs7525948 (nominal P < 0.05). In the logistic regression analysis of gene–gene interaction, a significant interaction effect of rs7525948 of DLGAP3 and rs2228622 of SLC1A1 (permutation P = 0.036) on SGA-induced OCSs was observed.
More recently, Cai et al. (2013) investigated the influence of three genes regulating glutamate transmission, i.e., SLC1A1, the glutamate receptor, ionotropic, N-methyl-D-aspartate 2B gene (GRIN2B), and the glutamate receptor, ionotropic, kainate 2 gene (GRIK2), on clozapine-induced OCSs in the Chinese population. The subjects included 250 clinically stable patients with schizophrenia receiving clozapine treatment, and they were divided into the OC group and the non-OC group based on almost the same criteria used in previous studies by our group (Kwon et al. 2009; Ryu et al. 2011). The authors observed trends of association of rs2228622 of SLC1A1 and rs890 of GRIN2B with clozapine-induced OCSs and a significant interaction effect of SLC1A1 and GRIN2B on this phenotype. They also reported the interaction effect of these two genes on the Y-BOCS score of the OC group.
Another replication trial of the association between SLC1A1 and SGA-induced OCSs was conducted in a European population mostly of German descent (Schirmbeck et al. 2012). Subjects included 103 schizophrenia patients treated with SGAs. The authors genotyped three SNPs including SNPs composing the risk haplotype identified in the previous study by Kwon et al. (2009). However, the positive finding observed in the previous study could not be reproduced, either in a single-marker analysis or in a haplotype analysis.
The discrepancy between study results might be due to ethnic differences and high vulnerability of these studies with a small sample size to a false-positive or false-negative result. In addition, differences in the phenotype definition, i.e., criteria for the OCS and non-OCS groups, between studies, and confounding effects of the medication type should be carefully considered. In the study by Schirmbeck and colleagues (Schirmbeck et al. 2012), the authors divided the patients into the OCS group and the non-OCS group based on the Y-BOCS score with the cutoff score of 8. Among the 103 subjects, 37.9 % of whom were treated with clozapine, 42.7 % (44/103) of subjects were diagnosed with SGA-induced OCSs (the OCS group). This rate was much higher compared to the rate of OCSs associated with SGAs (12.4 %) defined and identified in the previous study by our group (Lim et al. 2007) where a similar proportion (35.9 %) of the subjects were receiving clozapine. Regarding the medication type, all subjects were receiving clozapine in the study by Cai et al. (2013). In the previous study by Kwon et al. (2009), medication types (clozapine, olanzapine, or risperidone) were almost matched, showing no statistically significant difference between the OC and non-OC groups. However, in the study by Schirmbeck et al. (2012), there was a significant imbalance in medication types between the OCS and non-OCS groups. Thirty-seven out of 44 patients (84.1 %) of the OCS group were receiving clozapine or olanzapine, which are known to have a high OCS-inducing propensity (Schirmbeck et al. 2013). In the non-OCS group, a much smaller proportion of patients (22/59, 37.3 %) were receiving these drugs. In contrast, the rate of receiving amisulpride or aripiprazole, which were reported to have a low or no OCS-inducing propensity, was 4.5 % in the OCS group and 45.8 % in the non-OCS group. Although the authors included the medication type as a covariate in their analysis, it might not be sufficient to control for the strong imbalance effect of the medication type between groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


