Diagnostic Techniques
Endoscopy
Various methods can evaluate the gastrointestinal tract during pregnancy without reliance on x-ray techniques. Fiberoptic endoscopic instruments have revolutionized diagnosis and management of most gastrointestinal conditions, and these are particularly well suited for pregnancy. With endoscopy, the esophagus, stomach, duodenum, and colon can be inspected (Cappell, 2006, 2011). The proximal jejunum can also be studied, and the ampulla of Vater cannulated to perform endoscopic retrograde cholangiopancreatography—ERCP (Fogel, 2014; Kamani, 2012; Tang, 2009). Experience in pregnancy with videocapsule endoscopy for small-bowel evaluation is limited (Storch, 2006).
Upper gastrointestinal endoscopy is used for management as well as diagnosis of several problems. Common bile duct exploration and drainage are used for choledocholithiasis as described in Chapter 55 (p. 1096). It is also used for sclerotherapy as well as placement of percutaneous endoscopic gastrostomy (PEG) tubes. A number of concise reviews have been provided (Cappell, 2011; Fogel, 2014; Gilinsky, 2006).
Flexible sigmoidoscopy can be used safely in pregnant women (Siddiqui, 2006). In nonpregnant patients, colonoscopy is indispensible for viewing the entire colon and distal ileum for diagnosis and management of inflammatory bowel disease. Except for the midtrimester, reports of colonoscopy during pregnancy are limited, but preliminary results indicate that it should be performed if indicated (Cappell, 2010, 2011). Bowel preparation is completed using polyethylene glycol electrolyte or sodium phosphate solutions. With these, serious maternal dehydration that may cause diminished uteroplacental perfusion should be avoided.
Noninvasive Imaging Techniques
The obvious ideal technique for gastrointestinal evaluation during pregnancy is abdominal sonography. Because computed tomography (CT) use is limited in pregnancy due to radiation exposure, magnetic resonance (MR) imaging is now commonly used to evaluate the abdomen and retroperitoneal space (Khandelwal, 2013). One example is magnetic-resonance cholangiopancreatography—MRCP (Oto, 2009). These and other imaging modalities, and their safe use in pregnancy, are considered in more detail in Chapter 46 (p. 933).
 Laparotomy and Laparoscopy
Laparotomy and Laparoscopy
Surgery is lifesaving for certain gastrointestinal conditions—perforative appendicitis being the most common example. From the Swedish Registry database through 1981, abdominal exploration by laparotomy or laparoscopy was performed in 1331 of 720,000 pregnancies—approximately 1 in every 500 (Mazze, 1989). A similar incidence of 1 in 635 in nearly 50,000 pregnancies was described by Kort (1993). In both studies, the most common indications for surgery were appendicitis, an adnexal mass, and cholecystitis.
Laparoscopic procedures have replaced traditional surgical techniques for many abdominal disorders during pregnancy (Carter, 2004). In an updated report from the Swedish Registry database, there were 2181 pregnant women who underwent laparoscopy and 1522 who had laparotomy for nonobstetrical indications (Reedy, 1997). Use of these procedures was similar to their first study—approximately 1 in every 800 pregnancies. Both approaches were used usually before 20 weeks, and they were found to be safe. Long-term surveillance studies also suggest no deleterious effects for mother or fetus (Rizzo, 2003).
The most common nongynecological laparoscopic procedures performed during pregnancy are cholecystectomy and appendectomy (Fatum, 2001; Rollins, 2004). For more details and for descriptions of surgical technique, see Chapter 46 (p. 928) as well as Operative Obstetrics, 2nd edition (Gilstrap, 2002). Guidelines for diagnosis, treatment, and use of laparoscopy for surgical problems during pregnancy have been provided by the Society of American Gastrointestinal and Endoscopic Surgeons (Pearl, 2011).
 Nutritional Support
Nutritional Support
Specialized nutritional support can be delivered enterally, usually via nasogastric tube feedings, or parenterally with nutrition given by venous catheter access, either peripherally or centrally.
When possible, enteral alimentation is preferable because it has fewer serious complications (Bistrian, 2012; Hamaoui, 2003). In obstetrical patients, very few conditions prohibit enteral nutrition as a first effort to prevent catabolism. Even in extreme cases, such as recalcitrant hyperemesis gravidarum, percutaneous endoscopic gastrostomy with a jejunal port—PEG (J) tube—has been described (Saha, 2009).
The purpose of parenteral feeding, or hyperalimentation, is to provide nutrition when the intestinal tract must be kept quiescent. Central venous access is necessary for total parenteral nutrition because its hyperosmolarity requires rapid dilution in a high-flow vascular system. These solutions provide 24 to 40 kcal/kg/day, principally as a hypertonic glucose solution.
There have been a variety of conditions for which total parenteral nutrition has been employed during pregnancy (Table 54-1). Gastrointestinal disorders are the most common indication, and in the many studies cited, feeding duration averaged 33 days. It is imperative to emphasize that complications of parenteral nutrition are frequent, and they may be severe (Guglielmi, 2006). In an early report of 26 pregnancies, a 50-percent rate of complications, which included pneumothorax, hemothorax, and brachial plexus injury, was described (Russo-Stieglitz, 1999).
TABLE 54-1. Some Conditions Treated with Enteral or Parenteral Nutrition During Pregnancya
Achalasia
Anorexia nervosa
Appendiceal rupture
Bowel obstruction
Burns
Cholecystitis
Crohn disease
Diabetic gastropathy
Esophageal injury
Hyperemesis gravidarum
Jejunoileal bypass
Malignancies
Pancreatitis
Preeclampsia syndrome
Preterm labor/ruptured membranes
Short gut syndrome
Stroke
The most frequent serious complication is catheter sepsis, and Folk (2004) reported a 25-percent incidence in 27 women with hyperemesis gravidarum. Although bacterial sepsis is most common, Candida septicemia has been described (Paranyuk, 2006). The Centers for Disease Control and Prevention (2002) has published detailed guidelines to prevent catheter-related sepsis, and these have served to lessen the dangers of serious infections. Perinatal complications are uncommon, however, fetal subdural hematoma caused by maternal vitamin K deficiency has been described (Sakai, 2003).
There is also appreciable morbidity from long-term use of a peripherally inserted central catheter (PICC). Ogura (2003) reported infection with long-term access in 31 of 52 pregnant women. Holmgren (2008) reported complications in 21 of 33 women in whom a PICC line was placed for hyperemesis. Infections were the most common, and half of infected women also had bacteremia. From a review of 48 reports of nonpregnant adults, Turcotte and associates (2006) concluded that there were no advantages to peripherally placed catheters compared with centrally placed ones. Still, it seems reasonable for short-term nutrition—weeks—that PICC placement has a greater benefit-versus-risk ratio (Bistrian, 2012).
DISORDERS OF THE UPPER GASTROINTESTINAL TRACT
 Hyperemesis Gravidarum
Hyperemesis Gravidarum
Mild to moderate nausea and vomiting are especially common in pregnant women until approximately 16 weeks (Chap. 9, p. 187). In a small proportion of these, however, it is severe and unresponsive to simple dietary modification and antiemetics. In an attempt to quantify nausea and vomiting severity, Lacasse and colleagues (2008) have proposed a pregnancy-unique quantification of emesis and nausea (PUQE) scoring index. Severe unrelenting nausea and vomiting—hyperemesis gravidarum—is defined variably as being sufficiently severe to produce weight loss, dehydration, ketosis, alkalosis from loss of hydrochloric acid, and hypokalemia. Acidosis develops from partial starvation. In some women, transient hepatic dysfunction develops, and there is accumulation of biliary sludge (Matsubara, 2012). Other causes should be considered because hyperemesis gravidarum is a diagnosis of exclusion (Benson, 2013).
Study criteria have not been homogeneous, thus reports of population incidences vary. There does, however, appear to be an ethnic or familial predilection (Grjibovski, 2008). In population-based studies from California and Nova Scotia, the hospitalization rate for hyperemesis gravidarum was 0.5 to 0.8 percent (Bailit, 2005; Fell, 2006). Up to 20 percent of those hospitalized in a previous pregnancy for hyperemesis will again require hospitalization (Dodds, 2006; Trogstad, 2005). In general, obese women are less likely to be hospitalized for this (Cedergren, 2008).
The etiopathogenesis of hyperemesis gravidarum is likely multifactorial and certainly is enigmatic. It appears to be related to high or rapidly rising serum levels of pregnancy-related hormones. Putative culprits include human chorionic gonadotropin (hCG), estrogens, progesterone, leptin, placental growth hormone, prolactin, thyroxine, and adrenocortical hormones (Verberg, 2005). More recently implicated are other hormones that include ghrelins, leptin, nesfatin-1, and PYY-3 (Albayrak, 2013; Gungor, 2013).
Superimposed on this hormonal cornucopia are an imposing number of biological and environmental factors. Moreover, in some but not all severe cases, interrelated psychological components play a major role (Buckwalter, 2002; Christodoulou-Smith, 2011; McCarthy, 2011). Other factors that increase the risk for admission include hyperthyroidism, previous molar pregnancy, diabetes, gastrointestinal illnesses, some restrictive diets, and asthma and other allergic disorders (Fell, 2006; Mullin, 2012). The vestibular system has been implicated (Goodwin, 2008). An association of Helicobacter pylori infection has also been proposed, but evidence is not conclusive (Goldberg, 2007). And for unknown reasons—perhaps estrogen-related—a female fetus increases the risk by 1.5-fold (Schiff, 2004; Tan, 2006; Veenendaal, 2011). Finally, Bolin and coworkers (2013) reported an association between hyperemesis gravidarum and preterm labor, placental abruption, and preeclampsia.
Complications
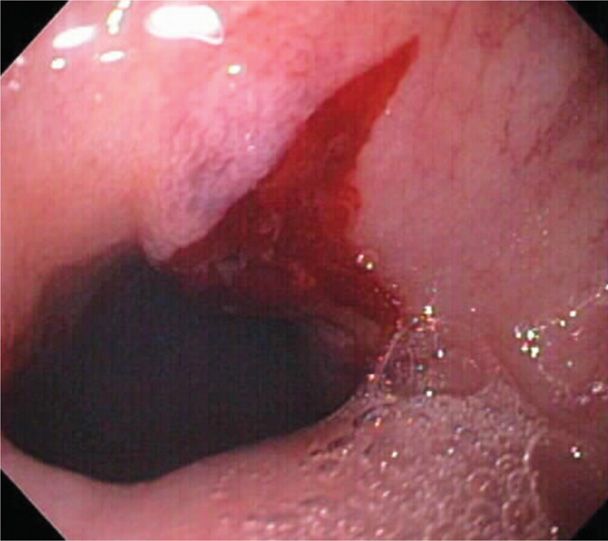
Vomiting may be prolonged, frequent, and severe, and a list of potentially fatal complications is given in Table 54-2. Various degrees of acute kidney injury from dehydration are encountered (Nwoko, 2012). We have cared for a number of women with markedly impaired renal function. The extreme example was a woman who required 5 days of dialysis when her serum creatinine level rose to 10.7 mg/dL (Hill, 2002). Complications from continuous retching include a Mallory-Weiss tear, such as that shown in Figure 54-1. Others are pneumothorax, pneumomediastinum, diaphragmatic rupture, and gastroesophageal rupture, which is Boerhaave syndrome (Chen, 2012; Schwartz, 1994; Yamamoto, 2001).
TABLE 54-2. Some Life-Threatening Complications of Recalcitrant Hyperemesis Gravidarum
Acute kidney injury—may require dialysis
Depression—cause versus effect?
Diaphragmatic rupture
Esophageal rupture—Boerhaave syndrome
Hypoprothrombinemia—vitamin K deficiency
Hyperalimentation complications
Mallory-Weiss tears—bleeding, pneumothorax, pneumomediastinum, pneumopericardium
Wernicke encephalopathy—thiamine deficiency
In more severe cases, plasma zinc levels are increased, copper levels decreased, and magnesium levels unchanged (Dokmeci, 2004). At least two serious vitamin deficiencies have been reported with hyperemesis in pregnancy. Wernicke encephalopathy from thiamine deficiency has been reported with increasing frequency (Di Gangi, 2012; Palacios-Marqués, 2012). In a review of 49 such cases, Chiossi (2006) reported that only half had the triad of confusion, ocular findings, and ataxia. With this encephalopathy, an abnormal electroencephalogram (EEG) may be seen, and usually there are MR imaging findings (Vaknin, 2006; Zara, 2012). At least three maternal deaths have been described, and long-term sequelae include blindness, convulsions, and coma (Selitsky, 2006). Last, vitamin K deficiency has been reported to cause maternal coagulopathy and fetal intracranial hemorrhage (Kawamura, 2008; Robinson, 1998; Sakai, 2003).
Management
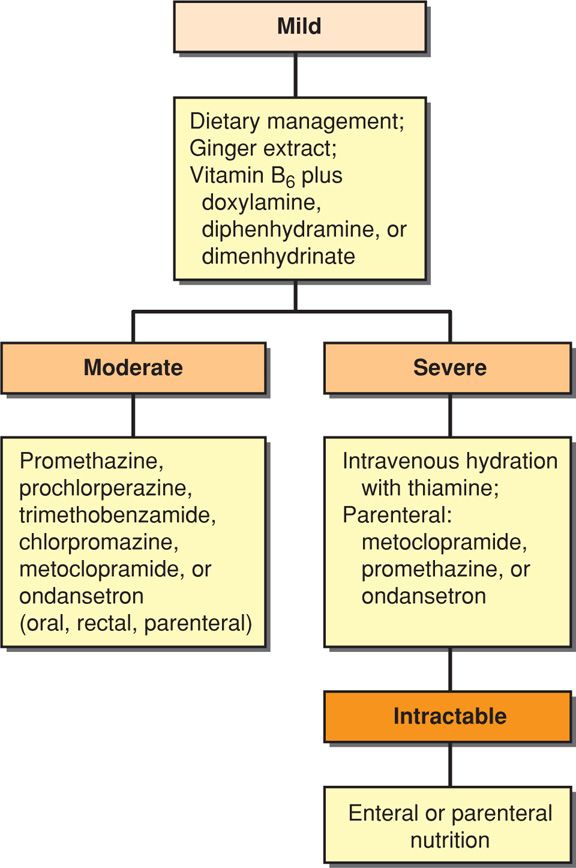
One algorithm for management of nausea and vomiting of pregnancy is shown in Figure 54-2. A Cochrane review reported a salutary effect from several antiemetics given orally or by rectal suppository as first-line agents (Jewell, 2000). The Food and Drug Administration (2013) recently approved Diclegis—a combination of doxylamine-pyridoxine—for morning sickness. When simple measures fail, intravenous Ringer lactate or normal saline solutions are given to correct dehydration, ketonemia, electrolyte deficits, and acid-base imbalances. There are no benefits to using 5-percent dextrose along with crystalloids (Tan, 2013). Thiamine, 100 mg, is given to prevent Wernicke encephalopathy (Niebyl, 2010).
If vomiting persists after rehydration and failed outpatient management, hospitalization is recommended (American College of Obstetricians and Gynecologists, 2013). Antiemetics such as promethazine, prochlorperazine, chlorpromazine, or metoclopramide are given parenterally. There is little evidence that treatment with glucocorticosteroids is effective. Two small trials found no benefits of methylprednisolone compared with placebo, but the corticosteroid-treated group had significantly fewer readmissions (Duggar, 2001; Safari, 1998). In another study from Parkland Hospital, Yost (2003) compared placebo with intravenous methylprednisolone plus two different tapered oral steroid regimens. They noted that a third in each group required readmission. In a study reported by Bondok (2006), pulsed hydrocortisone therapy was superior to metoclopramide to reduce vomiting and readmissions. Serotonin antagonists are most effective for controlling chemotherapy-induced nausea and vomiting (Hesketh, 2008). But when used for hyperemesis gravidarum, ondansetron was not superior to promethazine (Sullivan, 1996). Serotonin antagonist use in pregnancy is limited, but these drugs appear to be safe (Briggs, 2011).
With persistent vomiting after hospitalization, appropriate steps should be taken to exclude possible underlying diseases as a cause of hyperemesis. In one study, endoscopy did not change management in 49 women (Debby, 2008). Other potential causes include gastroenteritis, cholecystitis, pancreatitis, hepatitis, peptic ulcer, and pyelonephritis. In addition, severe preeclampsia and fatty liver are more likely after midpregnancy. And although clinical thyrotoxicosis has been implicated as a cause of hyperemesis, it is more likely that abnormally elevated serum thyroxine levels are a surrogate for higher-than-average serum hCG levels (Chap. 5, p. 103). This was termed “chemical hyperthyroidism” by Tan (2002). And of interest, Panesar and associates (2006) showed that a cohort of women with hyperemesis had lower serum thyrotropin levels. In our experiences, serum free thyroxine levels normalize quickly with hydration.
With treatment, most women will have a salutary response and may be sent home with antiemetic therapy. Their readmission rate is 25 to 35 percent in most prospective studies. If associated psychiatric and social factors contribute to the illness, the woman usually improves remarkably while hospitalized (Swallow, 2004). That said, symptoms may relapse in these women, and some go on to develop posttraumatic stress syndrome (Christodoulou-Smith, 2011; McCarthy, 2011). For some women, hyperemesis can be an indication for elective termination (Poursharif, 2007).
In the small percentage of women who continue to have recalcitrant vomiting, consideration is given for enteral nutrition. Vaisman (2004) described successful use of nasojejunal feeding for up to 21 days in 11 such women. Use of sonography to confirm correct placement of the tube has been described (Swartzlander, 2013). Percutaneous endoscopic gastrostomy with a jejunal port has also been reported (Saha, 2009; Schrag, 2007).
In our experiences, only a very few women will require parenteral nutrition (Yost, 2003). In a study of 166 women, Folk (2004) reported that 16 percent had central venous access established for nutrition. The litany of complications included line sepsis in 25 percent and thrombosis and infective endocarditis in one woman each.
 Gastroesophageal Reflux Disease (GERD)
Gastroesophageal Reflux Disease (GERD)
Reflux disease is seen in up to 15 percent of nonpregnant individuals (Kahrilas, 2012). Heartburn, or pyrosis, is especially common in late pregnancy, and it appears at some point in 50 to 80 percent of pregnancies (Mehta, 2010). The retrosternal burning sensation is caused by esophagitis from gastroesophageal reflux related to relaxation of the lower esophageal sphincter (Hytten, 1991). According to Costigan and colleagues (2006), common folklore is confirmed in that women with excessive heartburn do give birth to infants with more hair! Long-term complications are chronic esophagitis and adenocarcinoma.
Reflux symptoms usually respond to tobacco and alcohol abstinence, small meals, head of the bed elevation, and avoidance of postprandial recumbency and “trigger” foods. Oral antacids are first-line therapy. If severe symptoms persist, sucralfate is given with an H2-receptor antagonist such as cimetidine or ranitidine. If these are not successful, commonly used proton-pump inhibitors such as omeprazole or pantoprazole are also safe for use in pregnancy (Briggs, 2011; Mahadevan, 2006b). If there is still no relief, then endoscopy should be considered. Misoprostol is contraindicated because it stimulates labor (Chap. 26, p. 529).
For medical treatment failures in nonpregnant patients, surgical fundoplication is performed (Kahrilas, 2012). Although the procedure was not done during pregnancy, Biertho (2006) described 25 women who had undergone laparoscopic Nissen fundoplication before pregnancy. Only 20 percent had reflux symptoms during pregnancy.
 Hiatal Hernia
Hiatal Hernia
The older literature is informative regarding hiatal hernias in pregnancy. Upper gastrointestinal radiographs performed in 195 women in late pregnancy showed that 20 percent of 116 multiparas and 5 percent of 79 nulliparas had a hiatal hernia (Rigler, 1935). Of 10 women studied postpartum, hernia persisted in three at 1 to 18 months.
The relationship of hiatal hernia with reflux esophagitis, and thus symptoms, is not clear. One study demonstrated no relationship between reflux and hernia and showed that the lower esophageal sphincter functioned effectively even when displaced intrathoracically (Cohen, 1971). Nevertheless, during pregnancy, these hiatal hernias may cause vomiting, epigastric pain, and bleeding from ulceration. Schwentner (2011) reported severe herniation requiring surgical repair in a woman with a 12-week gestation. Curran (1999) described a 30-week pregnancy complicated by gastric outlet obstruction from a paraesophageal hernia.
 Diaphragmatic Hernia
Diaphragmatic Hernia
These are caused by herniations of abdominal contents through either the foramen of Bochdalek or that of Morgagni. Fortunately, they rarely complicate pregnancy. Kurzel and associates (1988) reviewed the outcomes of 18 pregnant women with such a hernia and who developed acute obstruction. Because the maternal mortality rate was 45 percent, they recommend repair during pregnancy even if a woman is asymptomatic. Herniation has been reported in one pregnant woman from a previous traumatic diaphragmatic defect and in another who had antireflux surgery in early pregnancy (Brygger, 2013; Flick, 1999). Several case reports also describe spontaneous diaphragmatic rupture from increased intraabdominal pressure during delivery (Chen, 2012; Ortega-Carnicer, 1998; Sharifah, 2003).
 Achalasia
Achalasia
A rare disorder, achalasia is a motility disorder in which the lower esophageal sphincter does not relax properly with swallowing. There is also nonperistaltic contraction activity of the esophageal muscularis to cause symptoms (Kahrilas, 2012; Khudyak, 2006). The defect is caused by inflammatory destruction of the myenteric (Auerbach) plexus within smooth muscle of the lower esophagus and its sphincter. Postganglionic cholinergic neurons are unaffected, thus, there is unopposed sphincter stimulation. Symptoms are dysphagia, chest pain, and regurgitation. Barium swallow radiography demonstrates bird beak or ace of spades narrowing at the distal esophagus. Endoscopy is performed to exclude gastric carcinoma, and manometry is confirmatory. If dilatation of the esophagus and medical therapy does not provide relief, myotomy is considered (Torquati, 2006).
During pregnancy, normal relaxation of the lower esophageal sphincter in those with achalasia theoretically should not occur. Even so, in most women, pregnancy does not seem to worsen achalasia. One report of 20 affected pregnant women found no excessive reflux esophagitis (Mayberry, 1987). Khudyak and coworkers (2006) reviewed 35 cases and described most women as symptom free, although esophageal dilatation was needed in a few. A maternal death at 24 weeks associated with perforation of a 14-cm diameter megaesophagus has been reported (Fassina, 1995).
Management of achalasia includes soft diet and anticholinergic drugs. With persistent symptoms, other options include nitrates, calcium-channel antagonists, and botulinum toxin A injected locally (Khudyak, 2006; Wataganara, 2009). Balloon dilatation of the sphincter may be necessary, and 85 percent of nonpregnant patients respond to this. Satin (1992) and Fiest (1993) and their associates reported successful use of pneumatic dilatation in pregnancy. One caveat is that esophageal perforation is a serious complication of dilatation. Spiliopoulos and colleagues (2013) described a 29-week pregnant woman with achalasia treated for 10 weeks with parenteral nutrition with surgical correction done postpartum.
 Peptic Ulcer
Peptic Ulcer
Erosive ulcer disease more often involves the duodenum rather than the stomach in young women. Gastroduodenal ulcers in nonpregnant women may be caused by chronic gastritis from H pylori, or they develop from use of aspirin or other nonsteroidal antiinflammatory drugs. Neither is common in pregnancy (McKenna, 2003; Weyermann, 2003). Acid secretion is also important, and thus underlies the efficacy of antisecretory agents (Suerbaum, 2002). Gastroprotection during pregnancy is probably due to reduced gastric acid secretion, decreased motility, and considerably increased mucus secretion (Hytten, 1991). Despite this, ulcer disease may be underdiagnosed because of frequent treatment for reflux esophagitis (Cappell, 1998; Mehta, 2010). In the past 45 years at Parkland Hospital, during which time we have cared for more than 500,000 pregnant women, we have encountered very few who had symptomatic ulcer disease. Before appropriate therapy was commonplace, Clark (1953) studied 313 pregnancies in 118 women with proven ulcer disease. They noted a clear remission during pregnancy in almost 90 percent. However, benefits were short lived. Symptoms recurred in more than half by 3 months postpartum and in almost all by 2 years.
Antacids are first-line therapy, and H2-receptor blockers or proton-pump inhibitors are safely prescribed for those who do not respond (Briggs, 2011; Diav-Citrin, 2005; Mahadevan, 2006b). Sucralfate is the aluminum salt of sulfated sucrose that inhibits pepsin. It provides a protective coating at the ulcer base. Approximately 10 percent of the aluminum salt is absorbed, and it is considered safe for pregnant women (Briggs, 2011).
With active ulcers, a search for H pylori is undertaken. Diagnostic aids include the urea breath test, serological testing, or endoscopic biopsy. If any of these are positive, antimicrobial therapy is indicated. There are several effective oral treatment regimens that do not include tetracycline and that can be used during pregnancy. These 14-day regimens include amoxicillin, 1000 mg twice daily along with clarithromycin, 500 mg twice daily; or metronidazole, 500 mg twice daily (Dzieniszewski, 2006; Mehta, 2010).
 Upper Gastrointestinal Bleeding
Upper Gastrointestinal Bleeding
In some women, persistent vomiting is accompanied by worrisome upper gastrointestinal bleeding. Occasionally, there is a bleeding peptic ulceration, however, most of these women have small linear mucosal tears near the gastroesophageal junction—Mallory-Weiss tears, as shown in Figure 54-1. Bleeding usually responds promptly to conservative measures, including iced saline irrigations, topical antacids, and intravenously administered H2-blockers or proton-pump inhibitors. Transfusions may be needed, and if there is persistent bleeding, then endoscopy may be indicated (O’Mahony, 2007). With persistent retching, the less common, but more serious, esophageal rupture—Boerhaave syndrome—may develop from greatly increased esophageal pressure.
DISORDERS OF THE SMALL BOWEL AND COLON
The small bowel has diminished motility during pregnancy. Using a nonabsorbable carbohydrate, Lawson (1985) showed that small bowel mean transit times were 99, 125, and 137 minutes in each trimester, compared with 75 minutes when nonpregnant. In a study cited by Everson (1992), mean transit time for a mercury-filled balloon from the stomach to the cecum was 58 hours in term pregnant women compared with 52 hours in nonpregnant women.
Muscular relaxation of the colon is accompanied by increased absorption of water and sodium that predisposes to constipation. This complaint is reported by almost 40 percent of women at some time during pregnancy (Everson, 1992). Such symptoms are usually only mildly bothersome, and preventive measures include a high-fiber diet and bulk-forming laxatives. Wald (2003) has reviewed treatment options. We have encountered several pregnant women who developed megacolon from impacted stool. These women almost invariably had chronically abused stimulatory laxatives.
 Acute Diarrhea
Acute Diarrhea
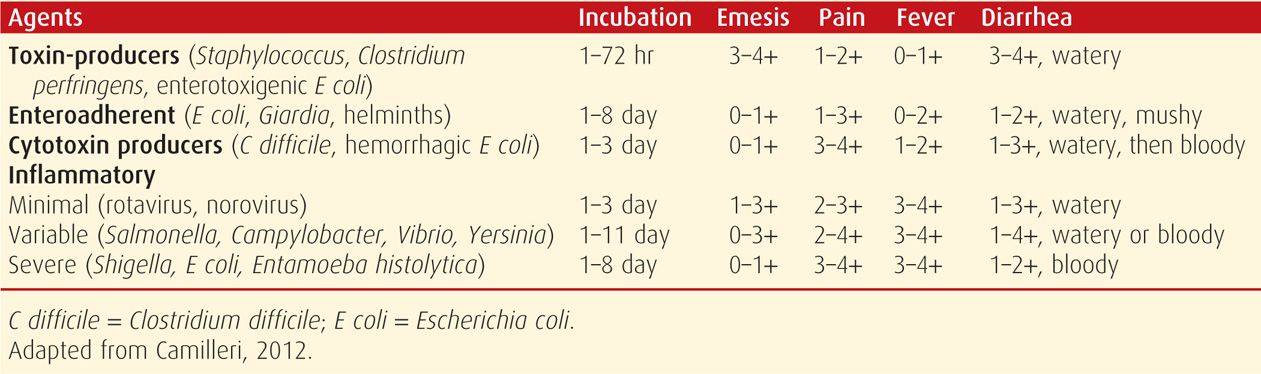
Most cases of acute diarrhea are caused by infectious agents. The large variety of viruses, bacteria, helminths, and protozoa that cause diarrhea in adults inevitably also afflict pregnant women. Some of these are discussed in Chapter 64. Evaluation of acute diarrhea depends on its severity and duration. According to Camilleri and Murray (2012), some indications for evaluation include profuse diarrhea with dehydration, grossly bloody stools, fever ≥ 38.5°C, duration of > 48 hours without improvement, recent antimicrobial use, immunocompromise, and new community outbreaks. Cases of moderately severe diarrhea with fecal leukocytes or gross blood may best be treated with empirical antibiotics rather than evaluation. Some features of the more common acute diarrheal syndromes are shown in Table 54-3.
The mainstay of treatment is intravenous hydration using normal saline or Ringer lactate with potassium supplementation in amounts to restore maternal blood volume and to ensure uteroplacental perfusion. Vital signs and urine output are monitored for signs of sepsis syndrome. For moderately severe nonfebrile illness without bloody diarrhea, antimobility agents such as loperamide may be useful. Bismuth subsalicylate may also alleviate symptoms.
Judicious use of antimicrobial agents is warranted. For moderate to severely ill women, some recommend empirical treatment with ciprofloxacin, 500 mg twice daily for 3 to 5 days. Specific pathogens are treated as needed when identified. Syndromes for which treatment is usually unnecessary include those caused by Escherichia coli, staphylococcus, Bacillus cereus, and Norwalk-like virus. Severe illness caused by Salmonella is treated with trimethoprim-sulfamethoxazole or azithromycin; Campylobacter with azithromycin; Clostridium difficile with oral metronidazole or vancomycin; and Giardia and Entamoeba histolytica with metronidazole (Mehta, 2010).
 Inflammatory Bowel Disease
Inflammatory Bowel Disease
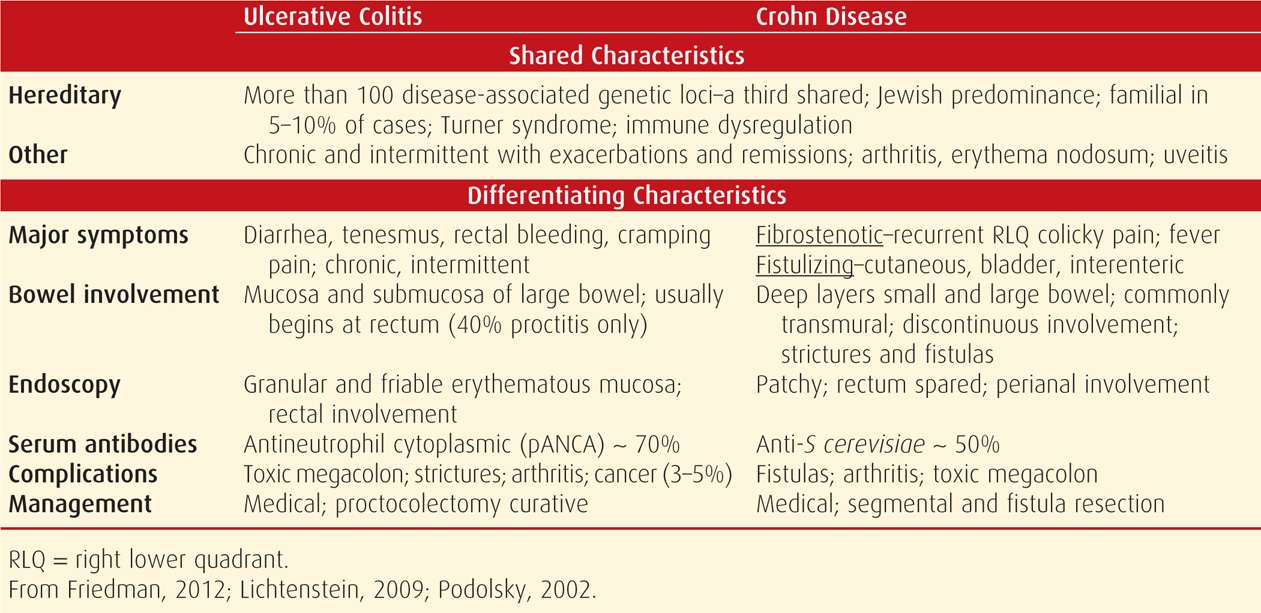
The two presumably noninfectious forms of intestinal inflammation are ulcerative colitis and Crohn disease. Differentiation between the two is important because treatment differs. That said, they both share common factors, and sometimes are indistinguishable if Crohn disease involves the colon. The salient clinical and laboratory features shown in Table 54-4 permit a reasonably confident diagnostic differentiation in most cases. The etiopathogenesis of both disorders is enigmatic, but there is genetic predisposition toward either. Inflammation is thought to result from dysregulated mucosal immune function in response to normal bacterial flora, with or without an autoimmune component (Friedman, 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree