Malassezia species are lipophilic yeasts and can cause catheter-related bloodstream infection in association with lipid emulsions.22,23 They require lipid supplementation in culture media and can be difficult to grow in the laboratory. Infections with the ubiquitous moulds Mucor, Rhizopus and Absidia (confusingly collectively termed zygomycosis or mucormycosis) are rare, but mortality is high. Most cases occur in pre-term infants and have a gastrointestinal or skin focus, unlike the sinopulmonary and rhinocerebral presentation seen in older immunocompromised hosts.24–26
16.3 Clinical features of Candida infections
The vast majority of IFIs are nosocomial, but infants can rarely present at or soon after birth with congenital candidiasis.
16.3.1 Congenital candidiasis
Most infants with congenital candidiasis are symptomatic at birth or soon afterwards, and infection is thought to come from maternal intrauterine infection or high-level vaginal colonization. The usual presentation is with a generalized eruption of erythematous macules, papules, and/or pustules that sometimes evolve to include vesicles and bullae (see Figure 13.1C).27 Extremely low birth-weight infants <;1000 g often present with a widespread desquamating and/or erosive dermatitis and often have had systemic candidiasis with a 40% mortality.27 Presence of an intrauterine foreign body, including a cervical stitch, is a predisposing factor for congenital cutaneous candidiasis in pre-term infants.27
Some infants with congenital cutaneous candidiasis also have respiratory distress and hepatosplenomegaly, suggesting ascending infection and possible haematogenous spread.28,29 Infants may rarely have fulminant disease without rash. In one report a 32-week gestation twin was well although neutropenic at birth but developed septic shock and died at 22 hours. The other twin did not develop a typical Candida rash (see Figure 13.1C) until day 3 of age.30 A form of congenital candidiasis affecting only the nails has been described.31
16.3.2 Candida dermatitis
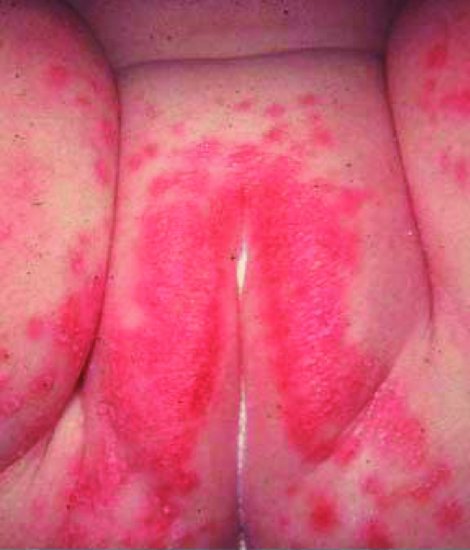
Most Candida dermatitis occurs in term infants and is benign. It particularly affects the diaper (napkin or nappy) region and is often associated with oral thrush and gastrointestinal colonization. However, damp and soiling are more common causes of diaper dermatitis and require barrier creams or topical steroids rather than antifungals.32,33 Candida classically causes a red, confluent, pruritic (itchy) rash with satellite lesions (Figure 16.2).
Candida dermatitis is more sinister in infants <;1500 g. In a prospective study 9 (32%) of 28 infants <;1500 g with mucocutaneous candidiasis developed invasive candidiasis despite topical antifungals.34 Candida skin lesions in infants <;1000 g are often more severe, with erythema, erosion, desquamation and draining wounds which crust and can bleed, sometimes called invasive fungal dermatitis, and associated with a high incidence of invasive infection.35,36 In a case-control study, affected infants were more likely than controls to have been delivered vaginally and to have received post-natal corticosteroids.35
16.3.3 Oral candidiasis
Oral candidiasis (thrush) is extremely common. There is a well-recognized association with antibiotics, particularly broad-spectrum antibiotics, but oral candidiasis is also common in infants who have not received antibiotics.37,38 Mild oral candidiasis causes white plaques on the tongue and buccal mucosa with few symptoms. Severe disease can cause painful mucosal erythema which may interfere with feeding. Infants of breastfeeding mothers can develop oral candidiasis and the mother may develop painful Candida infection of the nipple, both of which can interfere with breastfeeding until treated. Bottle-fed infants are more susceptible than breastfed infants to oral candidiasis.37,38
16.3.4 Systemic fungal infection
Candidaemic infants generally present with non-specific features indistinguishable from bacterial sepsis, including lethargy, feed intolerance, apnoea, increased need for respiratory support, abdominal distension, hyperglycaemia, temperature instability and hypotension.1,39–42 Infections can be fulminant with circulatory failure and shock.39–42 End-organ involvement is common, causing one or more of meningitis, chorioretinitis (see Figure 12.5) or endophthalmitis, renal abscesses (Figure 16.3), brain abscesses (Figure 16.4), endocarditis and hepatosplenomegaly.1,39 The clinical examination should include specialist eye examination with retinoscopy. Urine microscopy and CSF examination may reveal yeasts. Meningitis occurs in around 10–15% of all infants with Candida sepsis14,39,40 and the blood culture is negative in 50–60% of them.8,43 This shows that Candida can be difficult to grow from blood and that Candida meningitis will be missed in half of all infants if LP is not performed immediately.
Figure 16.3 Renal candidiasis: computerized tomographic (CT) scan showing filling deposits (arrows) in both kidneys due to renal lesions (fungal balls).
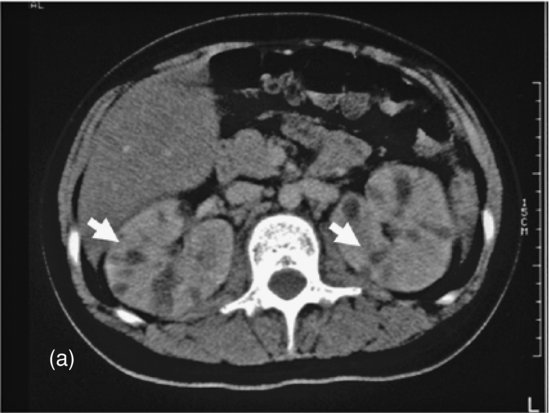
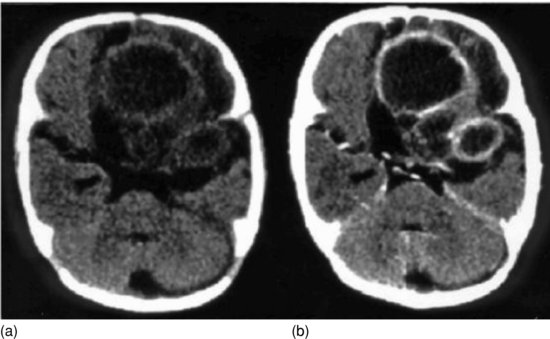
Figure 16.4 Cerebral candidiasis: CT scan unenhanced (a) and after iodine contrast (b). Shows rounded lesions with low-density centres and a ring-like periphery which enhances with contrast in the frontal and temporal regions of the left hemisphere. There is midline shift to the right.
The clinician would like to know whether a baby with suspected sepsis has IFI. Infants at increased risk of IFI (<;1500 g and infants who have had gastrointestinal surgery and major congenital malformations) are at even greater risk of bacterial infection. A maternal history of severe vaginal candidiasis or intrauterine foreign body is helpful but rare (Section 16.3.1). The most common age of presentation of invasive candidiasis is between 10 and 20 days when bacterial infection is also common but the range is from birth to over 100 days of age.8,36,44 A history of current or prolonged broad-spectrum antibiotic use may help. In one case series, 38% of infants with either C. albicans or C. parapsilosis infection and 67% with C. glabrata infection were on broad-spectrum antibiotics at the time of infection.44 In another series, infants with C. albicans or C. parapsilosis bloodstream infection, were more likely than infants with or CoNS bacteraemia to have been exposed to broad-spectrum antibiotics, systemic steroids and catecholamines, and 29 of 32 infants (90%) treated with third generation cephalosporins developed fungaemia compared with 5 of 19 (26%) not treated with third generation cephalosporins.45 Candida can cause central venous catheter-associated infections, but in an Israeli case series of infants <;1500 g with candidaemia, there was a strong association with hyperalimentation and lipid emulsions, but alimentation was delivered by peripheral venous catheters for all but one infant.5 This suggests that hyperalimentation fluids and lipids may be a more important risk factor than the type of catheter used.
Thrombocytopenia is common in both invasive fungal and bacterial infections (see Section 4.3.2). In an Italian study of septic infants <;1500 g, thrombocytopenia <;80×109/L occurred in 16% of infants with Gram-negative, 18% with Gram-positive (16% of CoNS) and 20% with fungal infections.46 In a US study, Gram-positive bacteria predominated as the cause of sepsis,47 whereas in India, Gram-negative sepsis predominated.48 Although most infants with fungal infections were thrombocytopenic in both sites, a clinically septic thrombocytopenic infant was far more likely to have bacterial sepsis.47,48
Benjamin et al. used a large database of 6172 infants <;1250 g to develop a clinical predictive model and generate a ‘candidaemia score’.49 In multivariate modelling, thrombocytopenia (<;150 × 109/L) and use of a third generation cephalosporin or carbapenem in the 7 days before the blood culture were the major risk factors for candidaemia in addition to the risk increasing with decreasing gestation. A candidaemia score of 2 or greater had a sensitivity of 85% and a specificity of 47% for predicting invasive candidiasis. The authors concluded that clinicians should consider empiric fungal therapy for thrombocytopenic infants <;25 weeks of gestation with suspected late-onset sepsis.49 However, the strength of this recommendation depends on the frequency of fungal infection compared with bacterial infection and the risks and benefits of antifungal therapy. It is less valid when the incidence of fungal infection is low.
16.3.5 Urinary tract Candida infection (renal candidiasis)
Candida grown from urine obtained by a freshly inserted catheter or suprapubic aspiration is considered diagnostic of a Candida urinary tract infection.50–53 There is a distinct syndrome of renal parenchymal candidiasis, presumed to arise by haematogenous seeding, which often coexists with hepatic and/or splenic Candida lesions (‘chronic disseminated’ or ‘hepatosplenic’ candidiasis).
In a study of 57 NICU infants with 60 urinary tract infections, 25 (42%) were caused by Candida, 13 of whom (52%) also had candidaemia, while renal pelvis fungal balls were present in 7 of 20 who had renal ultrasound scans.50 Renal fungus balls or renal fungal abscess were found in 13 of 41 infants with candiduria in another single-centre study, and the abnormality developed more than a week after the initial positive culture in 6 infants.51 Only 10 of 30 Canadian NICU infants with candiduria were <;30 weeks of gestation, while 10 had congenital heart disease. Fifteen of 26 tested had abnormal renal ultrasound scans and 4 infants developed disseminated candidiasis.53 Mortality and morbidity in infants <;1000 g with isolated candiduria is not significantly different from infants <;1000 g with candidaemia (Section 16.5),54 so confirmed candiduria in an infant <;1000 g should prompt full evaluation with cultures of blood and CSF and a renal ultrasound scan.54
About 5% of all infants with candidaemia have an abnormal renal ultrasound consistent with renal candidiasis.38 The investigation of an infant with proven candidaemia should, therefore, always include renal imaging, ideally with a renal ultrasound scan, although computerized tomography (CT) (Figure 16.4) may sometimes be necessary. In the above studies 12% and 20% of infants, respectively, with proven candiduria did not have any renal imaging performed.51,53
16.3.6 Candida eye infections
A meta-analysis of papers published from 1979 to 1992 found that Candida endophthalmitis occurred in 3% of infants with candidaemia.39 Candida chorioretinitis causes white or cream-coloured fluffy raised exudates, sometimes described as ‘snowballs’ (see Figure 12.6). Candidiasis can be unilateral or bilateral, can occur in the posterior fundus or the vitreous, rarely infects the lens and is often clinically silent. For this reason fundoscopy should be performed on all infants with possible IFI, and a formal ophthalmologic consultation is recommended for any infant with candidaemia, Candida meningitis or renal candidiasis. All Candida strains in Table 16.2 can cause Candida eye infections.
16.3.7 Intestinal candidiasis
Spontaneous intestinal perforation not due to NEC is an entity that often presents acutely with bluish discoloration of the abdominal wall.55–58 Invasive Candida infection of the intestinal wall is an important cause of spontaneous intestinal perforation. It often affects infants <;1000 g and has a high mortality.59 It is not clinically distinguishable from other forms of perforation and may be mistaken clinically for NEC.60 It is potentially treatable with antifungals and is an important diagnosis not to miss.
16.3.8 Candida meningitis and brain abscess
Candida meningitis occurs in 10–15% of all infants with candidaemia12,39,40 and brain abscess (Figure 16.4) or ventriculitis in 4% of candidaemic infants.39 Early fungal abscesses can be microscopic and not detected by ultrasound or CT, and LP may be normal.40,61,62
Most infants with Candida meningitis are <;1000 g and <;28 weeks of gestation. The median age of onset was 8 days in one study.63 The clinical manifestations of central nervous system Candida infections include bulging fontanelle, increased head circumference, seizures or focal neurologic signs but presentation may be non-specific.61–63 The CSF Gram stain is usually negative and there may be no white cells, while the CSF glucose is typically but not always low.39,40,61–63 Blood culture is negative in around 50% of infants with Candida meningitis,8,43 emphasizing the importance of LP in the diagnostic work-up of an infant with suspected late-onset infection and certainly one with proven candidaemia.
16.3.9 Candida endocarditis
Endocarditis is diagnosed in 5% of infants with candidaemia.39 Candida is the most common organism causing neonatal endocarditis after S. aureus.64 Most neonatal Candida endocarditis occurs in infants without congenital heart disease,65 and while there is a reported association with central venous catheters, such catheters are almost universal in extremely pre-term infants and many infants who develop endocarditis do not have a catheter in the right atrium at the time of diagnosis.65 Infants with Candida endocarditis often have persistently positive blood cultures.66 In a review of 30 reported cases of Candida endocarditis the mortality was 27%.65
16.4 Diagnosis
The diagnosis of fungal infections is based on clinical suspicion and supported by culture and sometimes radiology. A blood or CSF culture that grows Candida, even if it also grows a likely skin contaminant such as CoNS, should never be dismissed as a contaminant. Rapid tests such as PCR for fungal DNA67 and rapid tests to identify fungal mannan proteins in serum68 can potentially identify up to 90% of infants with candidaemia at the time of presentation, allowing timely empiric antifungal therapy. However, the relatively low sensitivity of these tests and the relatively low incidence of neonatal systemic fungal infection mean that routine use of the tests in suspected sepsis will result in large numbers of uninfected infants being treated with empiric antifungals. These tests are also expensive and not widely available, and PCR in particular is not well-standardized and relies on assays developed and validated locally, so inter-laboratory performance comparisons are generally lacking.
16.5 Treatment
Some authorities have argued that empiric antifungal therapy should be started or at least strongly considered for those infants with suspected late-onset sepsis who are at greatest risk of IFI.49,69 One proposal is to consider starting empiric antifungals for thrombocytopenic infants <;25 weeks of gestation with suspected late-onset sepsis.49 Another proposal is to use prophylactic antifungals for infants <;1000 g and consider empiric antifungal therapy for thrombocytopenic infants 1000–1500 g with negative blood cultures who are not responding to anti-bacterial antibiotics after 48 hours.69 Finally, empiric antifungal treatment could be initiated in infants who have a positive fungal PCR67 or positive mannan test68 (Section 16.4). None of these approaches has been formally evaluated and, while they may appear attractive initially, it is important to know how many infants will receive empiric fungal infection for every one with true fungal infection. Furthermore, unless cultures subsequently become positive, it may be difficult to know when to stop antifungal therapy once started.
16.5.1 Antifungal treatment of invasive fungal infection
The agents most commonly used and most studied in neonatal fungal infections are amphotericin B and fluconazole.
Amphotericin B is a polyene macrolide antifungal which increases cell membrane permeability by binding to ergosterols in the fungal membrane leading to cell death. Amphotericin B is active against Candida species except C. lusitaniae (Table 16.2) and is active against Aspergillus and the zygomycetes.70 While nephrotoxicity and systemic reactions are major problems when using conventional amphotericin B in cancer patients, conventional amphotericin B is surprisingly well-tolerated in neonates71–73 and although nephrotoxicity can occur, it rarely limits treatment. In older children and adults, liposomal amphotericin is often used to decrease toxicity and improve tissue penetration, notably to the CSF. However, CSF penetration of conventional amphotericin is much higher in pre-term neonates (40–90% vs. 2–4% of blood levels).71 A retrospective review of invasive Candida infections in US neonatal units from 1997 to 2003 found an overall mortality of 19%, but the mortality was considerably higher in infants receiving liposomal amphotericin (29%) than in those receiving conventional amphotericin (18%) (OR 2.0, 95% CI 1.2–3.3) or fluconazole (16%) (OR 2.4, 95% CI 1.2–4.8).74 Although this was a retrospective study, not an RCT, the data suggest extreme caution in the use of liposomal amphotericin, which incidentally is far costlier than conventional amphotericin. Liposomal amphotericin should not be used to treat neonates unless RCT evidence becomes available showing it is superior to conventional amphotericin B or fluconazole. A small test dose of amphotericin B used to be recommended for neonates in case of allergic reactions, as is recommended for adults, but since allergic reactions have not been described in neonates, test doses are no longer recommended. The dose of conventional amphotericin B is 0.5–1 mg/kg/day IV once daily.71–73
Fluconazole is a triazole antifungal which inhibits a fungal cytochrome P450 enzyme involved in ergosterol synthesis by the cell membrane. Fluconazole has excellent CSF penetration, is less nephrotoxic than amphotericin B although hepatotoxicity has been described,75 and resistance can develop with prolonged or extensive use.76,77 While most studies of fluconazole prophylaxis have not shown selection of azole-resistant fungi, in one study from India77 fluconazole prophylaxis for 6 years was associated with a predominance of non-albicans Candida species, predominantly C. glabrata, which is often resistant to fluconazole (Table 16.2). The treatment dose of fluconazole in neonatal fungal infection is 12 mg/kg/day IV or oral once daily, and a loading dose of 25 mg/kg has been recommended on pharmacokinetic grounds.78,79 In a small study of 10 infants an initial loading dose of 25 mg/kg IV was safe and achieved therapeutic levels within 24 hours, quicker than without a loading dose.80
There is a dearth of RCTs of antifungals in neonatal systemic fungal infections.81 A small RCT from South Africa compared conventional amphotericin B and fluconazole for 23 infants with disseminated fungal sepsis, 21 with Candida species and 2 with Rhodotorula rubra.82 The case fatality was high: 4 of 12 (33%) for fluconazole and 5 of 11 (45%) for amphotericin B, (p > 0.05). The fluconazole group did not need central lines, whereas three babies on amphotericin B did, and intravenous therapy was given for significantly shorter time to the fluconazole group. The safety profiles were similar. It has been argued that if fluconazole is being used for prophylaxis, amphotericin B should be used for empiric antifungal treatment.69
The echinocandins (micafungin, caspofungin and anidulafungin) inhibit fungal β-(1,3)-D-glucan synthase, interfering with cell wall biosynthesis. The echinocandins are active against Candida and Aspergillus (although fungistatic against Aspergillus) but inactive against zygomycetes.69,83 All three echinocandins have been used in neonates,83 particularly micafungin84 but also anidulafungin85 and caspofungin.86 A small RCT from Egypt which compared caspofungin and amphotericin B reported no significant difference in mortality, although caspofungin was superior in terms of a poorly defined endpoint of a ‘favourable response’.87 In the absence of adequate efficacy and safety data, echinocandins should not be used as first-line antifungal therapy, although salvage therapy use is understandable.83
Flucytosine (5-flucytosine, fluorocytosine) is a fluorine analogue of cytosine which inhibits DNA synthesis when converted to 5-fluorouracil. It is active against all Candida species except C. krusei, but resistance develops rapidly if it is used as monotherapy (Table 16.2). It has excellent CSF penetration and is well absorbed orally and excreted through the kidneys, so has been used in combination therapy of renal candidiasis and of Candida meningitis. However, there is no objective evidence that its use improves outcome compared to monotherapy with amphotericin B or fluconazole. There is considerable individual variation in serum concentrations and clearance after oral doses.71 The recommended dose is 25–100 mg/kg/day orally once daily, and the dose should be modified based on drug levels.71
16.5.2 Duration of antifungal treatment
There are no RCTs on duration of antifungal treatment, but fungi are slow-growing organisms that are difficult to eradicate and prolonged treatment is necessary. On the basis of limited evidence from case series, most authorities recommend systemic therapy for 14–21 days after negative cultures. Amphotericin B has traditionally been given for 3–4 weeks. Candida meningitis may relapse and prolonged therapy for 4–6 weeks is advised. For susceptible Candida species initial IV therapy can be changed to oral fluconazole when the infant is improving and oral feeds are tolerated. Six weeks of parenteral therapy is recommended for endocarditis. Isolated renal candidiasis can be treated with 7 days of therapy but infants with fungal balls or undrainable abscesses should be treated until there is ultrasonographic or radiographic resolution.
16.5.3 Central venous catheter removal in invasive fungal infection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree