Introduction
Why?
A major aim of prenatal diagnosis is to provide parents with relevant information on the health of the fetus as early as possible during the pregnancy. Sonographic measurement of the nuchal translucency (NT) thickness at 11–13 weeks is a well-established screening method for aneuploidies1 and other major anomalies.2 Other noninvasive methods are already being used to identify aneuploidies,3 and they will probably replace nuchal scanning for this purpose in the near future. At that point, the main goal of the 11–13 week scan will be to identify major structural anomalies in the fetus.
The most likely site of such anomalies is the fetal heart, which is already fully developed at this stage of gestation.4 Effective screening and accurate diagnosis of cardiac malformations is already feasible during the 12th week of gestation.5–7 Several markers of aneuploidy, including increased NT,8 tricuspid valve regurgitation (TR),9 and abnormal flow through the ductus venosus (DV),10 have also proved to be effective in identifying congenital cardiac anomalies. With the aid of new tools—e.g., genomics arrays, which can identify submicroscopic deletions, duplications, or other rearrangements11—early detection and diagnosis of major cardiac malformations in the fetus can provide parents with prognostic information that will enable them to make timely, informed choices about the management of the pregnancy.12
Who?
The first-trimester examination of the fetal heart begins—and usually ends—with the US practitioner. If the suspicion of cardiac abnormalities arises during the screening examination, however, the patient must be referred to a pediatric cardiologist for a specific diagnosis and prognostic assessment. Later, if the decision is made to interrupt the pregnancy, a key role will also be played by the pathologist, whose job it is to confirm and shed additional light on the antenatal diagnosis. For the latter two figures, earlier assessment of the fetal heart has some obvious drawbacks. The size of the organ at 11–13 weeks (cross sectional diameter 4–6 mm) is clearly a challenge for the cardiologist, who must diagnose the specific abnormality and assess the fetus’ prognosis, but studies have shown that over 70% of major congenital heart diseases (CHDs) can be defined early with this method.13–18 For the pathologist, the obstacles are even more daunting since dissection of an organ this size is out of the question.
For US practitioners, however, the objective is merely to screen the fetus for signs of possible cardiac abnormalities during the NT scan. In their view, the first-trimester cardiac examination is a way to add diagnostic value to a well-accepted screening program without significantly increasing its costs. Studies have demonstrated that, in approximately 90% of all low-risk cases, the addition of fetal cardiac screening prolongs the routine NT scan by no more than ten minutes or so, and the rate of false-positive results is <5%.19,20
A high index of suspicion at screening is still the major determinant of successful detection of major cardiac defects at this stage of gestation. The presence of an increased NT can predict 30% of the major CHDs, and the percentage increases if this marker is also associated with TR or abnormal DV flow.21 Single-marker positivity is much more common, however, and early fetal echocardiography will probably not be available for all patients with findings of this type. Consequently, US practitioners themselves should be able to provide additional information based on a basic study of the fetal heart aimed at distinguishing cases that require prompt attention by a pediatric cardiologist.
What?
Screening of the fetal heart during the NT scan should include verification of all of the following elements:
The pulmonary arterial branches, the pulmonary veins, and the systemic veins, which are examined during the mid-gestational study, are not assessed during the first-trimester examination. The order in which the above elements are assessed will vary: be flexible and seize the moment! If the fetal position is obstructing your view of one structure, examine something else until the position changes. Each component should be examined and the findings documented photographically in the report.
When?
Ideally, the first-trimester cardiac examination is done during the NT scan, between the 11th and 13th weeks of gestation, when crown–rump lengths (CRLs) range from 46–84 mm, and the heart itself has a cross-sectional diameter of 4–6 mm. The optimal period for assessing NT and the fetal heart is about the 12th week. During this period, CRLs range from 55–65 mm, and the fetus frequently assumes a transverse, dorsoposterior position that greatly facilitates visualization of the nuchal area and the heart. Later, a vertical position is more likely. With the technology currently being used by most practitioners, it is undeniably easier to examine the fetal heart at 13 instead of 12 weeks. However, if this approach is used, only a fraction of the patients who come in for NT scanning will benefit from the early cardiac screening.
How?
For US practitioners familiar with second-trimester cardiac screening and diagnosis, the main obstacles to a successful first-trimester examination are technical. Equipment settings for the second-trimester cardiac study are well known, and most scanners come with a preinstalled program (or preset) for this examination. In contrast, settings for the study of the first-trimester fetal heart have to be configured by the operator. For those practitioners who are put off by the fact that there is no single “magic” button to press, this chapter should be of particular value: it will provide indispensable information on the technical aspects of cardiac imaging that supplements information provided elsewhere in this book. The image quality offered by a given ultrasound system is the result of a unique balance between the specific features of the scanner and the transducers, so any exam preset that is offered is inevitably machine-specific. Understanding the specific goals and problems of early fetal cardiac imaging allows operators to choose equipment more effectively and to adjust the settings to obtain the best images possible in each case.
Technical aspects: equipment
Sonographic studies of fetal heart disease were initially attempted by skilled obstetricians using the transvaginal route.23–25 Over the years, continuous improvements in US resolution have made it possible to carry out these studies with a transabdominal approach. This advance enabled cardiologists to perform a complete evaluation of the fetal heart that included diagnosis and prognostic assessment of abnormalities.
NT studies are usually performed during transabdominal scans, which are not only more acceptable to patients than the transvaginal route: they are also less dependent on the position of the fetus, do not require manipulations, and can be mastered more rapidly with a more affordable training period.
Ultrasound image resolution depends on several factors, including the composition of the body wall and the depth of the structure being examined. Thus far, no studies have been conducted that specifically examined the position of the fetus in the first trimester. Research carried out by our group has shown that the hearts of fetuses between the ages of 11 and 13 weeks lie anywhere from 4–11 cm below the maternal skin surface (Fig. 5.1).
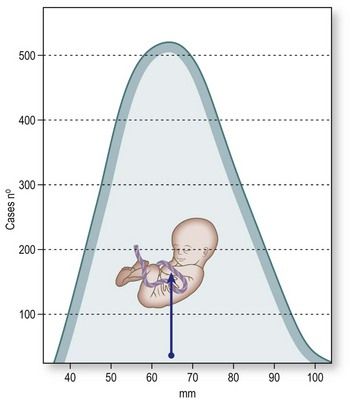
Figure 5.1 Distribution of fetal heart depths at the time of the nuchal scan (based on studies of 1388 fetuses) with a median CRL of 60 mm (range 46–74 mm). Depths, expressed as distance from the maternal skin surface to the crux of the fetal heart, ranged from 40–110 mm (median 63 mm).
According to the model we studied, the actual depth will be 4–6 cm in about 60% of the cases and up to 11 cm in the others. At this stage, the depth of the fetal heart is not significantly influenced by the maternal BMI (as it is at mid or late gestation) because at 12 weeks, the transducer is placed over the symphysis pubis, where the thickness of the fat layer is limited. Problems are more likely to arise from the presence of a retroverted uterus (Fig. 5.2).
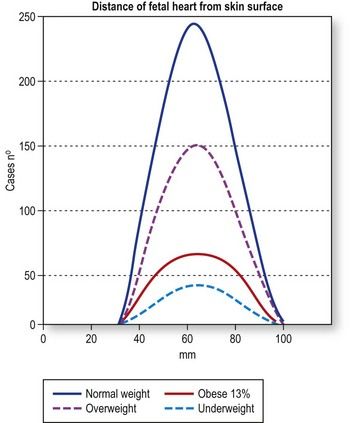
Figure 5.2 Distribution of fetal heart depths as a function of maternal BMI (based on the study of 1388 fetuses at 12 weeks of gestation). The number of fetuses studied is admittedly quite limited, but on the basis of this experience, there do not appear to be significant differences in the depth of the fetal heart in mothers who are underweight, normal weight, overweight, and obese.
The transabdominal probes used by obstetricians are usually curved-array transducers designed for second-trimester imaging, which requires a wide field of view and a penetration depth of up to 20 cm. Each ultrasound beam emitted radiates at a 90° angle from the face of the transducer, and if the face is convex, the beams diverge progressively with increasing imaging depth. Consequently, the distance between adjacent beams increases with the distance from the transducer, and resolution deteriorates as a function of imaging depth (Fig. 5.3).26 The image quality provided by curved-array transducers is acceptable during a second-trimester study of the fetal heart, which is relatively large, but high resolution is essential for examining the tiny anatomical features of the first-trimester heart. With conventional convex-array transducers, resolution begins to diminish substantially at a depth of 6.5 cm (Fig. 5.4). This decline will affect visualization of the heart, which lies >6.5 cm below the surface in approximately 40% of all 12-week fetuses (Fig. 5.5).
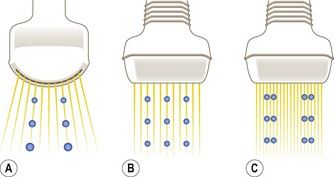
Figure 5.3 (A) With a conventional convex transducer (6 MHz), image resolution diminishes with distance because of the divergence of the ultrasound beams. (B) With a linear array transducer of the same frequency (6 MHz), the beams travel parallel to one another, and there is no loss of resolution with increasing depth. (C) Higher frequency linear transducers (12 MHz) can be used to obtain higher resolution images of the same area.
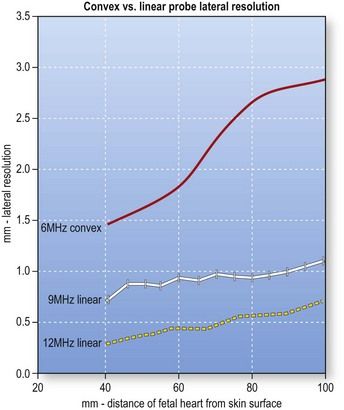
Figure 5.4 Lateral resolution (i.e., the capacity for distinguishing two adjacent points) offered by conventional convex and linear array transducers as a function of imaging depth (based on examination of phantom models). The areas studied lay at depths of 4–11 cm (where the fetal heart is expected to be found at 12 weeks). Both convex and linear transducers provide adequate lateral resolution for imaging structures approximately of 6–7 cm below the maternal skin surface, but at greater depths the resolution of the 6 MHz convex transducer (red line) decreases progressively. In contrast, with a 9 MHz linear probe, resolution remains stable (white line), and it is further enhanced by use of higher frequency linear probes (12 MHz) (yellow dotted line)
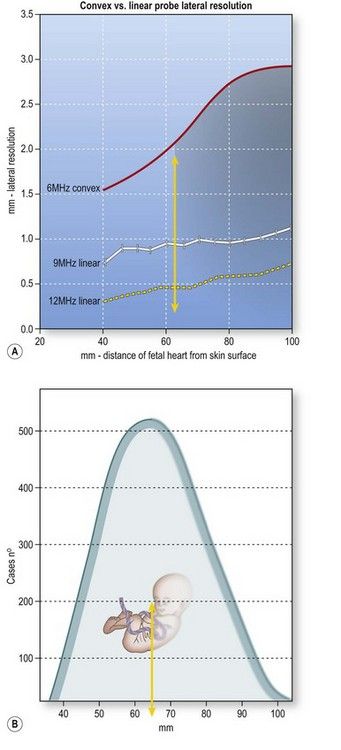
Figure 5.5 (A) With conventional convex-array transducers, resolution begins to decline at a depth of 6–7 cm and therefore affects optimal visualization of the heart (shaded area). (B) According to the model we studied, the hearts of approximately 40% of all 12-week fetuses will be in this area (shaded area).
For this reason, I recommend using a linear transducer, which maintains high spatial resolution with distance. It can also be used at higher frequencies than convex-array transducers. For examining features like the AV-valve offset (which measures 0.2 mm in a 12-week fetus), the difference is impressive (Fig. 5.6). With recently developed linear-array systems, one can shift back and forth between a linear format (when higher resolution is required) and a virtual trapezoidal format (similar to that of a curvilinear transducer) when a wider field of view is needed (Fig. 5.7). The efficiency of high-frequency linear transducers has improved dramatically in recent years. Advances in crystal design and piezoelectric materials, improved pulse shape and disposition, and increases in the number of elements have greatly enhanced penetration and image resolution.27,28
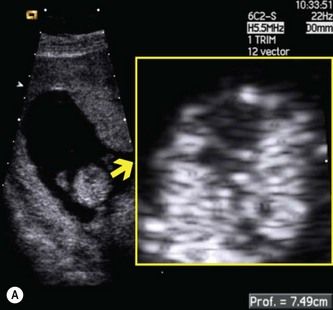
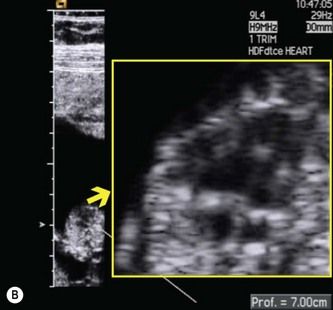
Figure 5.6 Resolution of traditional convex and linear transducers in a 12-week fetus. The fetal heart lies 7 cm below the maternal skin surface, and the AV-valve offset at this age is only 0.2 mm. (A) When the study is done with a 6 Mhz convex probe, the loss of spatial resolution makes it impossible to assess the AV-valve offset. (B) When the same fetus is imaged with a 9 Mhz linear probe, the AV valve offset is clearly visible.
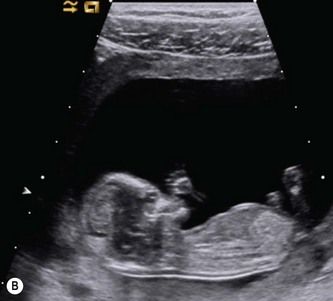
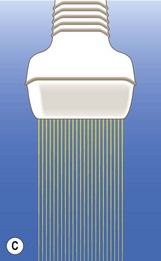
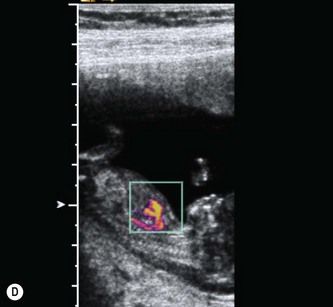
Figure 5.7 Use of linear array transducers at 11-13 weeks. (A) Modern linear array transducers can be used in a virtual trapezoidal format, which resembles that of a convex-array transducer. (B) Most US practitioners are more comfortable using a wide field of view for imaging the fetus. (C) The switch to linear format is done in real time. (D) Higher B-mode and color flow anatomical details will be available at all depths.
Currently available transducers used for a first-trimester fetal heart scan should have frequency range of 6–12 Mhz and an effective penetration depth of 12 cm. Power management techniques can be used to design special pulses that enhance penetration. We have successfully used coded excitation (i.e., coded pulse trains) in our studies. The effects resemble that of a power increase, but this approach has no impact on the mechanical index.29,30 Coded excitation allows high-frequency (9–12 MHz) linear transducers to compensate for the natural decrease in penetration caused by the attenuation of the ultrasound signal. Structures at depths of up to 11 cm—typical for the first-trimester fetus—can thus be clearly depicted. Coded signals are also used to amplify low-intensity waves produced by blood cells and allow B-mode-based visualization of flow (B-flow) (Fig. 5.8).
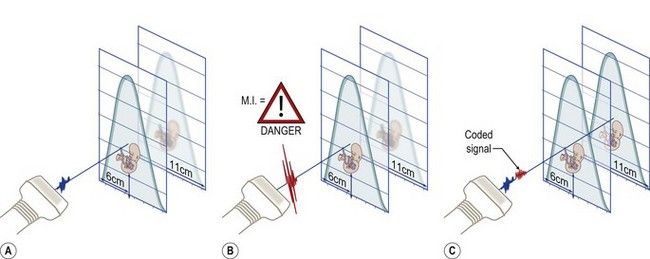
Figure 5.8 Higher frequencies translate into higher image resolution. A general consequence of increasing the imaging frequency is that the depth of penetration decreases because high frequencies are subjected to greater attenuation than lower frequencies. (A) A high-frequency linear transducer (9–15 Mhz) usually has an effective penetration depth of up to 6–7 cm, which is adequate for its intended purpose, i.e., the study of small parts. (B) Penetration can be enhanced by increasing the acoustic power, but in medical imaging, and fetal imaging in particular, the rule of thumb is to keep the power or the mechanical index (MI) as low as possible. (C) Another option is to apply more power to the ultrasound pulse along the length of its path, i.e., increase the pulse duration. This is the principle underlying coded excitation. With this technique, the effective penetration of a high-frequency linear probe can be extended. Coded signals can also be used to amplify low intensity waves produced by blood cells in the vessels and provide visualization of flow based on B-mode (B-flow).
Reverberation artifacts are common in a moving small liquid-filled areas like those of the fetal heart. They can be overcome by using a scanner equipped for the use of the following techniques:
Tissue harmonic imaging (THI)
THI suppresses the weak echoes caused by artifacts. Compared with fundamental-frequency imaging, it reduces reverberation noise, improves border delineation, and increases spatial, axial, and contrast resolution.31 For early studies of the fetal heart, recent generation THI technology should be used whenever possible: ongoing development of this technology has dramatically improved spatial resolution in the near-medium field of view, where the fetus is located at the time of the NT scan (Fig. 5.9).32
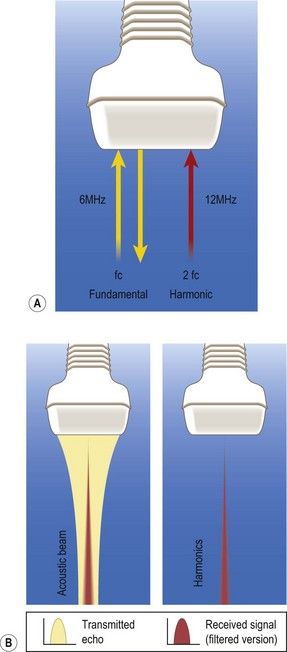
Figure 5.9 (A) In conventional B-mode imaging (or fundamental imaging), the ultrasound beam is transmitted and received over the same frequency bandwidth. However, reflected echoes in human tissue generate higher-frequency components (the tissue harmonic response) whose directional performance and spatial resolution are better than those of the fundamental wave because their frequency is twice as high and they pass through the tissue only once, reducing the number of overlapping signals. (B) Because the second harmonic component has much less energy than the fundamental component, it can be picked up only by a high-sensitivity receiving system with a broad dynamic range. Harmonic imaging constructed with the pulse-inversion technique can dramatically improve resolution.
Compound imaging
Spatial compounding allows one to view a structure from different angles and combine the information thus acquired to obtain a single sharper image. The temporal resolution is somewhat diminished because the frame displayed is the average of several sweeps, but this loss is offset by increased contrast resolution and reduced acoustic noise,33 which improves border definition. In some cases, spatial compounding can be used with tissue harmonics to better demonstrate the AV-valve offset (approximately 0.2 mm) and septoaortic continuity in the fetal heart (Fig. 5.10).
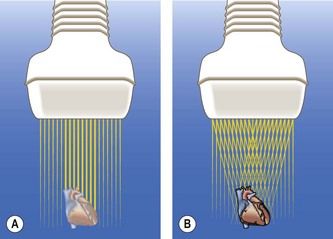
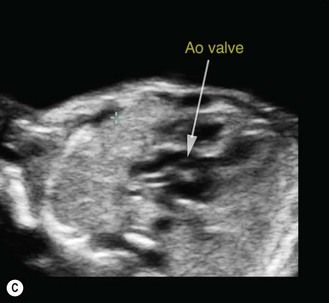
Figure 5.10 (A) In conventional modality, the ultrasound beam is transmitted at a 90° angle from the face of the transducer. (B) With compound imaging, the ultrasound beams are transmitted from several different angles and are thus associated with different artifact patterns. When frames do not overlap, the patterns are considered artifacts and suppressed; where frames overlap, the patterns are considered real structures and reinforced. It is geometrically obvious that a linear-array transducer will be able to generate more beams from different angles than a curved-array transducer. Spatial compounding enhances contrast resolution and border definition. (C) Compounding can be useful for assessing specific cardiac features, like septoaortic continuity. As this modality considerably reduces the frame rate, its use at first trimester is limited.
Post-processing
Different post-processing settings should be tested to minimize speckle artifacts, ultrasound signals caused by variations in the positions and signal strength of the various scattering within the beam. The first-trimester heart has all the features of a structure that produces speckle. Not all speckle is noise: some represents true tissue information. The ultrasound machine should be able to distinguish between the two and eliminate only the useless, artifactual subset.34,35
Post-processing settings designed to make a sense of speckle signals have been marketed under different names. Optimizing these functions to reduce noise and improve conspicuity requires patience, perseverance, and, ideally, the assistance of an application specialist. The best image quality is achieved by fine tuning.
Technical aspects: settings
Image quality reflects the characteristics of the transducer, those of the ultrasound machine, and the balance between the two components. General aspects of the exam presets are described in Chapter 2, which should be consulted before reading this chapter. Settings for the first-trimester fetal heart study should be defined by the practitioner, ideally with the aid of an application specialist.
To create a first-trimester 2D package, the operator should image a fetus with a CRL of 6 cm whose heart is no more than 4–6 cm beneath the maternal skin surface. The examination begins with an apical four-chamber view of the heart, and spatial resolution should be optimized to allow demonstration of the AV-valve offset while viewing the crux of the heart. Noise suppression should then be adjusted so that the image of a ventricular chamber includes no spurious signals (Fig. 5.11).
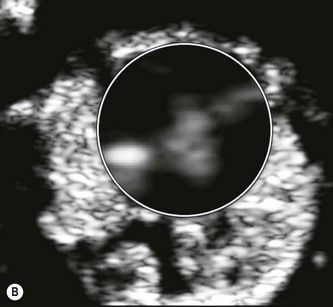
Figure 5.11 (A) Heart chambers contain no spurious signals. (B) Crux of the heart: the AV valve offset at 12 weeks is 0.2 mm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


