High risk
Cyclophosphamide
Ifosfamide
Melphalan
Busulfan
Intermediate risk
Cisplatin with low cumulative dose
Carboplatin with low cumulative dose
Adriamycin
Low risk
Actinomycin D
Vincristine
Methotrexate
5.3 Female Fertility
Female fertility requires a functioning HPG axis, ovaries, and a uterus. Reproductive potential however, is mainly limited by available oocytes. The process of oogenesis begins before birth with a peak in oocyte number at 20 weeks gestation (6–7 million), followed by progressive atresia and a quantitative oocyte drop to 1–2 million at birth, followed by 300,000 at menarche (Gracia and Woodruff 2012; Knopman et al. 2010). In normal menstruating women, ovarian function depends on pituitary gonadotropin production. Secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. With each cycle, multiple follicles are selected to enter the growing pool and begin to mature, but only one will become the dominant follicle selected for ovulation, while the remaining follicles undergo atresia (Gracia and Woodruff 2012; Knopman et al. 2010). The dominant follicle produces estradiol, which triggers an LH surge and results in the mature oocyte being released into the fallopian tube where fertilization occurs. Follicle development and atresia is a continuous process occurring throughout the reproductive lifespan. When a healthy woman reaches her mid to late 30s, a threshold number of follicles are reached at which reproductive potential drops significantly, follicular depletion accelerates, and the remaining oocytes are of overall poorer quality. Finally, with continued decline of ovarian reserve, a second threshold occurs when a woman is in her late 40s or early 50s, the start of menopause, when it is no longer possible to have biologic children (Gracia and Woodruff 2012).
5.3.1 Effects of Chemotherapy and Radiation on Female Fertility
Chemotherapy and radiation therapy destroy ovarian follicles, and predispose treated females to premature ovarian failure. The negative effect of chemotherapy on female fertility is dependent on the age of the patient at the time of treatment, the specific chemotherapeutic agent(s) used, and the cumulative dosing (Gracia and Woodruff 2012; Trudgen and Ayensu-Coker 2014). Similar to males, alkylating agents are the most gonadotoxic to female fertility. In contrast to males, significantly higher doses of alkylators are required to cause infertility in females. However, gonadotoxicity in females is more age dependent than males. Specifically, older age has a greater negative impact, as there is an overall smaller follicular pool during cancer therapy (Ginsberg 2011). The natural depletion of the number of follicles present in an individual female’s ovaries can be accelerated by cancer treatment. If the degree of depletion is near complete, then the result is acute ovarian failure defined by early menopause with consequent infertility that occurs during or shortly after treatment (Gracia and Woodruff 2012). If the degree of depletion is more moderate, then the individual is at risk for premature menopause, where females remain fertile following cancer therapy but have an overall shortened reproductive life span.
Radiation to the ovaries, either through direct pelvic radiation, abdominal or spine radiation, or scatter radiation, in doses as low as 1–2 Gy in girls and 4–6 Gy in adult females can have permanent negative effects on the ovaries by causing depletion of follicles (Gracia and Woodruff 2012). Irradiation may also impact fertility by causing damage to the uterine musculature and vascular structures, thereby limiting the ability of the survivor to carry a pregnancy to term. High dose cranial irradiation in the dose range of 35–40 Gy or greater, can cause hypogonadism through effects on the hypothalamus and pituitary. This exposure results in dysregulation of hormonal pathways responsible for menstruation and fertility.
Finally, the surgical resection of reproductive organs has obvious implications for infertility when hysterectomies or oophorectomies become necessary (See Table 5.2).
Table 5.2
Gonadotoxic radiation dosage used in pediatric bone tumors
High risk |
Total body irradiation for bone marrow transplant/stem cell transplant |
Pelvic or whole abdominal radiation dose ≥6 Gy in adult women |
Pelvic or whole abdominal radiation dose ≥10 Gy in postpubertal girls |
Pelvic or whole abdominal radiation dose ≥15 Gy in prepubertal girls |
Intermediate risk |
Testicular radiation dose I–6 Gy from scattered pelvic or abdominal radiation |
Pelvic or whole abdominal radiation dose 5–10 Gy in postpubertal girls |
Pelvic or whole abdominal radiation dose 10–15 Gy in prepubertal girls |
Craniospinal radiotherapy dose ≥25 Gy |
5.4 Pediatric Bone Tumor Experience
Most patients with aggressive pediatric bone tumors will receive a treatment regimen that includes high doses of chemotherapy with alkylating agents and other chemotherapeutic drugs that can impair fertility, aggressive surgical resection, and sometimes radiation therapy. Reproductive outcomes in childhood cancer survivors have not been studied extensively with large cohorts of patients, and most information comes from small single-institutional studies.
5.4.1 Childhood Cancer Survivor Study (CCSS)
The primary exception from small studies is the Childhood Cancer Survivor Study (CCSS) which reported on multiple outcomes for cancer survivors including late mortality, subsequent malignant neoplasms, chronic health conditions, fertility, and health status using matched sibling controls (Ginsberg et al. 2010b). Childhood cancer survivors from the CCSS were half as likely as their matched control siblings to sire a pregnancy with an overall reported rate of premature ovarian failure of 6 % (Chemaitilly et al. 2006; Larsen et al. 2003; Longhi et al. 2000, 2003). High risk exposures identified in the study included radiation greater than 7.5 Gy to the testes and high doses of alkylating agents including cyclophosphamide dose greater than 7.5 g/m (Green et al. 2010). In fact, when controlled for these factors, survivors were equally likely as their matched control siblings to sire a pregnancy (Green et al. 2010).
5.4.2 Ewing Sarcoma
Three hundred forty-one survivors of Ewing sarcoma (EWS) were separately analyzed within this cohort for their fertility status. Infertility was common among both male and female EWS survivors. Female survivors were 35 % less likely to report a pregnancy than siblings of childhood cancer survivors, even after excluding women who were surgically sterile (Ginsberg et al. 2010b). Similarly, male EWS survivors were 62 % less likely to report siring a child than male siblings (Ginsberg et al. 2010b). Contemporary therapy for EWS includes high dose cyclophosphamide and ifosfamide, so we can anticipate that the rate of infertility will not be much different for patients treated today. Another 58 patient analysis of sarcoma patients including 22 with EWS reviewed semen analyses before, during, and after treatment with chemotherapy with multi-agent chemotherapy including cyclophosphamide (Meistrich et al. 1992). While pretreatment levels were similar to the control subjects, azoospermia occurred within 4 months of treatment (Meistrich et al. 1992). The cumulative dose of cyclophosphamide was the most significant determinant of recovery to normospermic levels; approximately 70 % of those who had received doses less than 7.5 g/m2 recovered, but only 10 % recovered when doses exceeded 7.5 g/m2 (Meistrich et al. 1992). In a retrospective study of 17 male sarcoma survivors treated with high dose cyclophosphamide within a multi-agent chemotherapy regimen, a total dose of cyclophosphamide of 7.5 g/m2 was also associated with a decreased risk of an abnormal sperm count with an incidence of azoospermia of 58.8 % (Kenney et al. 2001). This analyses also demonstrated that exposure prior to the onset of puberty did not appear to protect males from subsequent gonadal damage. All of the patients exposed to 25 g/m2 of cyclophosphamide or higher were azoospermic irrespective of their pubertal status at the time of treatment (Kenney et al. 2001).
5.4.3 Osteosarcoma
In patients with osteosarcoma, exposure to Ifosfamide has also been analyzed in several case series. Kenney et al. reviewed six patients with non-metastatic osteosarcoma who were treated with multi-agent chemotherapy (Kenney et al. 2001). They reported their results as consistent with previously reported studies that used semen analysis as their measure of spermatogenesis, demonstrating 52–89 % of survivors with abnormal sperm counts after treatment with Ifosfamide 24–45 g/m2 (Kenney et al. 2001). The United Kingdom Children’s Cancer Study Group (UKCCSG) reviewed gonadal function of patients who were treated for Ewing or soft tissue sarcoma with regimens containing ifosfamide as the only potential gonadotoxic agent (Williams et al. 2008). Male patients who received high dose Ifosfamide (greater than 60 g/m2) showed 73 % to be subfertile and 27 % azoospermic (Williams et al. 2008). Hormone profiles were consistent with germ cell failure, 31 % had increased FSH, while Leydig cell function remained preserved (Williams et al. 2008). The female cohort for the study had different treatment regimens including ifosfamide (Longhi et al. 2003). Unfortunately, very few patients were willing to undergo semen analysis and/or hormone testing, so the study was only able to conclude that Ifosfamide seems to be a major cause of infertility in male osteosarcoma patients with a dose-dependent relationship between higher doses of ifosfamide with higher probability of becoming sterile (Longhi et al. 2003). They also noted that Cisplatin causes azoospermia or oligospermia with a gradual recovery of spermatogenesis in 50 % of patients after 2 years and 80 % after 5 years following cumulative dose of 600 mg/m2 (Longhi et al. 2003). In contrast, the Rizzoli Institute also reviewed the reproductive function of female survivors of localized osteosarcoma. Twenty-four of their 92 patients were prepubertal at the time of chemotherapy and underwent menarche at a mean age of 13 (range 11–16), consistent with the mean age of menarche of the normal population in Italy (Longhi et al. 2000). Of the postpubertal patients, 69 % experienced amenorrhea during chemotherapy, 66/68 patients resumed menstrual activity by the end of chemotherapy, whereas the 2 patients age 39 and 43 at the start of treatment had permanent amenorrhea (Longhi et al. 2000). Of the 92 patients, only 2 patients reported a willingness to conceive without achieving a pregnancy and 20 others had successful pregnancies with healthy offspring (Longhi et al. 2000). A Finnish study demonstrated similar findings in a small cohort of female osteosarcoma survivors with all patients having amenorrhea at the completion of chemotherapy, but 90 % had some returned menses within 3–12 months of follow-up (Wikstrom et al. 2003). The three patients who had irregular menses cycles upon resumption of menstruation represented the highest cumulative dose of Ifosfamide (54.5 g/m2, 63.5 g/m2, and 60.4 g/m2) (Wikstrom et al. 2003). Importantly, resumption of menses does not directly correlate to fertility and pregnancy, and thus is a limitation of this small study.
5.5 Clinical Guidelines
Several organizations have published guidelines for fertility preservation in cancer patients. The ethics committee of the American Society for Reproductive Medicine produced the first guideline on fertility preservation and reproduction in 2005. It addressed the roles of cancer specialists and fertility specialists in the counseling and referral of cancer patients including safety and efficacy of options, special considerations for minor patients, and ethical considerations in the disposition of stored gametes, embryos, and gonadal tissue (Robertson et al. 2005). The American Society of Clinical Oncology (ASCO) followed with their guideline for practicing oncologists in 2006, which was updated in 2013 (http://www.asco.org/guidelines/fertility). The American Academy of Pediatrics published a guideline in 2008 (Fallat and Hutter 2008) that is currently being revised for publication, reviewing the technical aspects of fertility preservation in pediatric and adolescent patients as well as the ethical considerations. Specifically, the AAP recommendations are as follows:
Evaluation of candidacy for fertility preservation should involve a team of specialists, including a pediatric oncologist and/or radiation oncologist, a fertility specialist, an ethicist, and a mental health professional.
1.
Cryopreservation of sperm should be offered whenever possible to male patients or families of male adolescents.
2.
Current fertility-preservation options for female children and adolescents should be considered experimental and are offered only in selected institutions in the setting of a research protocol.
3.
In considering actions to preserve a child’s fertility, parents should consider a child’s assent, the details of the procedure involved, and whether such procedures are of proven utility or experimental in nature. In some cases, after such consideration, acting to preserve a child’s fertility may be appropriate.
4.
Instructions concerning disposition of stored gametes, embryos, or gonadal tissue in the event of the patient’s death, unavailability, or other contingency should be legally outlined and understood by all parties, including the patient if possible.
5.
Concerns about the welfare of a resultant offspring with respect to future cancer risk should not be a cause for denying reproductive assistance to a patient.
5.6 Risk Identification
Once the definitive cancer diagnosis is made, fertility risk identification may proceed as the proposed treatment plan unfolds. As previously discussed, fertility risk factors include age, chemotherapeutic agents used, radiation dose, and possible surgical interventions. Oncologists have many risk assessment tools at their disposal. The NCCN guidelines for Adolescent and Young Adult Oncology recommend the risks to fertility be discussed prior to the initiation of chemotherapy and a referral for fertility preservation occur within 24 h for who choose to pursue it (Coccia et al. 2014). It also refers providers to the on-line risk assessment tool at www.livestrong.org/we-can-help/fertility-services.
Based on these recommendations, Fig. 5.1 shows an overview of the stratification of fertility considerations based on planned anti-cancer therapy and Fig. 5.2 is an algorithm for screening new oncology patients for fertility counseling and referrals.
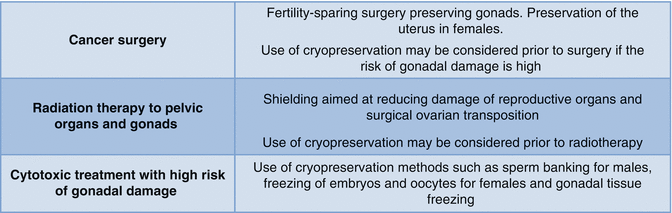
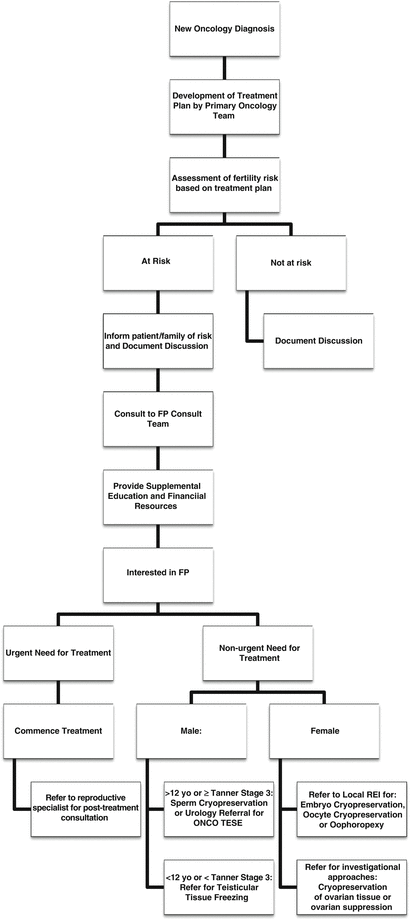

Fig. 5.1
Fertility preservation options to consider based on treatment

Fig. 5.2
Work-flow algorithm for fertility preservation during cancer therapy
5.7 Pretreatment Options for Males
Cryopreservation of ejaculated semen is the recommended fertility preservation method for adult males and pubertal boys. Mature spermatozoa can be found at Tanner III stage with a testis volume above 5 ml, although 20 % of males at Tanner stage II or above with testicular volumes greater than 10–12 ml have achieved spermiation, with the ability to provide sperm for cryopreservation (Gracia and Woodruff 2012). Cryopreservation of two to three samples collected following a period of abstinence of 48 h between samples is recommended prior to starting treatment to ensure optimal DNA integrity and sperm quality (Gracia and Woodruff 2012; Rodriguez-Wallberg and Oktay 2014; Leonard et al. 2004).
In cases of ejaculation failure, sperm may be extracted via testicular sperm extraction (TESE), a surgical procedure where sperm is extracted via an open incision through the scrotum and one or multiple biopsies of testicular tissue are obtained and sperm can be harvested and placed in media for analysis and cryopreservation (Quinn and Vadaparampil 2012; Schrader et al. 2003). Patients with neurologic compromise can undergo vibrostimulatory ejaculation if the sacral reflex is intact. In the absence of an intact spinal pathway, electroejaculation can be performed, although this requires anesthesia if the patient does not have a complete spinal cord injury (Gracia and Woodruff 2012). In electroejaculation, a rectal probe is used to transmit electrical stimulation to the short postsynaptic sympathetic fibers in the walls of the ejaculatory organs leading to ejaculation (Berookhim and Mulhall 2014).
Collecting sperm prior to cancer therapy has a unique set of challenges. Patients are facing cancer or other serious medical issues. The context in which patients are asked to provide specimens is fraught with stress. They are often feeling ill, emotionally overwhelmed, are in pain, and are possibly under the influence of narcotics or other medications. They may be hospitalized and have limited privacy for masturbation. Patients may be unable to perform under severe time constraints and pressure, particularly adolescent boys, and there may be religious or personal objections to masturbation that are difficult to overcome (Ogle et al. 2008). Every attempt should be made to facilitate the collection of ejaculated sperm. Patients should be provided private and relaxing physical conditions whenever possible. It is important to avoid discussing the details of sample collection with adolescents in the presence of their families. Separate discussions with the adolescent patient and family members help avoid introducing unnecessary embarrassment that might present yet another barrier to successful collection. Sexually stimulating audiovisual materials, such as magazines or videos, should be provided to the patient if appropriate.
Testicular tissue cryopreservation is the only available option in prepubertal patients who have not yet initiated spermatogenesis. Investigators are examining cryopreservation of testicular tissue through either cell suspension or whole tissue as a possible option for fertility preservation (Gracia and Woodruff 2012). The tissue can be obtained via testicular biopsy. Although prepubertal germ cells do not contain mature spermatozoa, they do demonstrate the presence of spermatogonial diploid stem cells, which maintain the capacity to differentiate into mature cells given the appropriate microenvironment. Investigators from the Children’s Hospital of Philadelphia published reports of prepubertal boys who underwent testicular biopsy with tissue cryopreservation (Ginsberg et al. 2010a). Despite this and other similar experimental protocols reporting preservation of prepubertal tissue, no study to date has demonstrated a technique to transform this immature, cryopreserved tissue into functional gametes either in vivo or in vitro (Ginsberg et al. 2010a). Furthermore, hypothetical risks associated with tissue preservation exist. Given the underlying malignancy in patients undergoing testicular tissue extraction, there is concern regarding the potential for reseeding the cancer when cryopreserved tissue is reintroduced into the native host. Thus, testicular tissue cryopreservation is performed strictly under an experimental, IRB-approved protocol, as no clinically proven means to use such tissue for reproductive purposes exists at this time (Gracia and Woodruff 2012) (See Table 5.3).
Table 5.3
Fertility preservation options for males
Definition | Pubertal status | Time requirement | Success rates | Cost | Timing | Special considerations | |
|---|---|---|---|---|---|---|---|
Sperm banking (masturbation) | Sperm is obtained through masturbation, then frozen | After puberty | Outpatient procedure | Generally high The most established technique for men | Approx. $1,500 for 3 samples; storage fees average $300–$500/year | Before treatment | Deposits can be made every 24–48 h. May consider TESE or electroejaculation if male unable to ejaculate |
Radiation shielding of gonads | Use of shielding to reduce the dose of radiation delivered to the testes | Before and after puberty | In conjunction with radiation treatments | Possible with select radiation fields and anatomy | Generally included in the cost of radiation treatments | During treatment | Expertise required; does not protect against effects of chemotherapy |
Testicular tissue freezing | Tissue obtained through biopsy and frozen for future use | Before and after puberty | Outpatient procedure | No available human success rates | $500–$2,500 for surgery; $300–$1,000 for freezing; $300–$500/year for storage | Before treatment | May be only option for prepubescent boys. Experimental use with IRB approved protocol |
Testicular sperm extraction (TESE) | Use of biopsy to obtain individual sperm from testicular tissue | Tanner stage II | Outpatient procedure | 30–70 % in postpubescent patients | $4,000–$16,000 (in additional to costs for IVF) | Before or after treatment | Center should be able to freeze sperm found at the time of biopsy
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|