Introduction
As the trend to delay childbearing continues among women in the United States and other developed countries, the demand for effective methods of fertility preservation has grown considerably. Perhaps the most pressing need for such technology applies to women facing imminent loss of ovarian function due to impending cancer therapy or other causes of premature ovarian failure. Though many of the techniques to preserve future fertility are still considered experimental, several options show great promise and will be discussed in this chapter.
Age-related decrease in female fertility
The progressive loss of oocytes that occurs from fetal life until menopause is one of the defining features of the age-related decline in female fertility. The oocyte pool peaks while the female fetus is in utero, reaching approximately 6–7 million oocytes at 20 weeks’ gestation. Subsequently, progressive atresia occurs so that the number of remaining oocytes is approximately 1–2 million at birth and 200,000 at the onset of puberty. During the reproductive years, there is continued atresia, which occurs at an accelerated rate after the age of 37 in normal women. The average age of menopause is 51, at which time there are approximately 1000 oocytes remaining.
As the number of oocytes declines over time, their quality also declines and eventually reaches a critical threshold below which pregnancy is no longer possible. The decrease in quality primarily refers to an increased prevalence of aneuploid oocytes with age, largely due to dysfunctions of the meiotic spindle. Errors in meiotic segregation result in higher rates of chromosomally abnormal embryos, translating into higher rates of spontaneous abortion and lower chances of pregnancy. It is estimated that the prevalence of aneuploid oocytes approaches 100% after the age of 45. For women who are approaching advanced reproductive age but are not ready to become pregnant or are not in the position to have a child, options to preserve fertility including embryo and oocyte cryopreservation may be considered.
Cancer therapy-induced decrease in female fertility
The need for fertility preservation options is particularly pertinent for women facing imminent loss of ovarian function due to cancer therapy. Over 600,000 women are diagnosed with cancer each year in the United States. Thousands of these women are in their reproductive years and many of them have not yet started or completed their families. Steady advances in cancer treatment have greatly improved survival rates. Unfortunately, the most successful treatment strategies, which include chemotherapy and radiation, often cause infertility and premature menopause, thus rendering many female cancer survivors incapable of having future children.
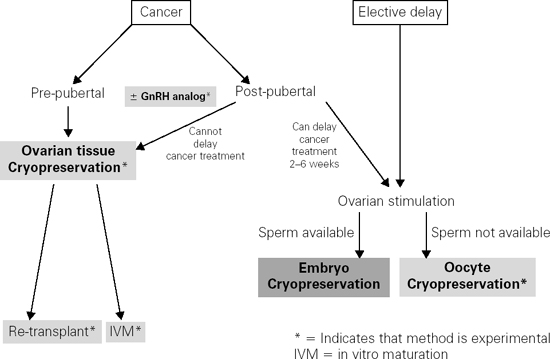
Ovarian follicles are remarkably vulnerable to agents that cause DNA damage, including ionizing radiation and chemotherapy. These treatments result in a dramatic reduction in follicular and oocyte reserve due to apoptosis, and eventually lead to ovarian atrophy. With respect to radiation, the degree of oocyte depletion depends on the field of treatment, total dose, and fractionation schedule. Ovarian damage resulting from cytotoxic chemotherapy depends on the dose administered and the agent used, with alkylating agents such as cyclophosphamide and ifosfamide posing the greatest risk of infertility. For both forms of cancer therapy, patients who are younger at the time of treatment are less likely to suffer infertility and have later onset of premature menopause than patients who are older at the time of treatment. Overall, the incidence of impaired fertility is not well characterized due to variable definitions, but is reported to range between 15% and 90%. Thus, the potential for infertility is significant and is a known source of stress for female cancer survivors. Therefore, discussion about options for fertility preservation is critical. The algorithm presented in Figure 105.1 should aid the clinician in counseling patients about their options, which are described in further detail below.
Options for fertility preservation
Embryo cryopreservation
Embryo freezing is the most proven method of fertility preservation and is not considered experimental. Since by definition an embryo is a fertilized egg, this process requires sperm. It is an excellent option for women who have a male partner or are interested in using donor sperm. It involves ovarian stimulation with daily injectable gonadotropins, which typically starts at the beginning of a menstrual period and continues for approximately 10–14 days to mature the eggs. Alternative approaches to stimulation include use of tamoxifen or aromatase inhibitors, which can be employed if exposure to high estrogen levels is a concern (as in breast cancer patients). The eggs are removed by a transvaginal ultrasound-guided needle (a procedure which is done under conscious sedation) and are then combined with the sperm in the laboratory. The resulting embryos can be stored indefinitely until the patient is ready to use them. The entire process takes 2–6 weeks to complete, and therefore requires that the patient can safely delay cancer therapy for this amount of time. Pregnancy rates from frozen-thawed embryos depend on the age of the woman at the time of the egg retrieval, ranging from approximately 20% at age 40 to 40% at age 35 per embryo transfer.
While embryo freezing is the standard method of fertility preservation and has been successful in routine use for about two decades, it is only applicable in postpubertal female patients and those who have a current male partner or are willing to consider sperm donation.
Oocyte cryopreservation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree