2.0 mg: Bedol® (ReSource Medical), Elleste-Solo 2 mg® (Meda), Zumenon® (Abbott Healthcare)
1.0 mg: Femoston-conti® 1 mg/5 mg and Femoston® 1 mg/10 mg (both Abbott Healthcare), Angeliq® (Bayer), Elleste-Duet ®1 mg (Meda), Kliovance® and Novofem® (both Novo Nordisk)
2.0 mg: Clinorette® (ReSource Medical), Elleste-Duet® 2 mg (Meda), Femoston® 2 mg/10 mg (Abbott Healthcare), Nuvelle® continuous (Bayer), Kliofem® and Trisquens® (both Novo Nordisk)
2.0 mg: Climagest® 2 mg or Climesse® (both Novartis), Cyclo-Progynova® (Meda), Indivina® 2 mg/5 mg and Tridestra® (both Orion)
0.625 mg: Premique® and Prempak C® 0.625 (both Pfizer)
1.25 mg: Prempak C® 1.25 (Pfizer)
(17ß-estradiol in all cases)
0.040 mg: Elleste-Solo MX® (Meda)
0.050 mg: Estraderm MX 50® and Estradot 50® (both Novartis), Evorel 50® (Janssen), FemSeven 50® (Merck Serono), Progynova TS 50® (Bayer)
0.075 mg: Estraderm MX 75® and Estradot 75® (both Novartis), Evorel 75® (Janssen), FemSeven 75® (Merck Serono)
0.100 mg: Estraderm MX 100® and Estradot 100® (both Novartis), Evorel 100® (Janssen), FemSeven 100® (Merck Serono), Progynova TS 100® (Bayer)
Hormone replacement therapy with natural estrogens
Nomenclature is always a problem when referring to steroid hormones – think of the difficulties that came with the use of “generations” when referring to progestogens. The word “natural” is used in this chapter to describe steroids with estrogenic activity that can be found in living organisms (humans, other animals, plants). The route by which a specific steroid is produced is immaterial: a steroid may have been developed from plant (soy or yam) or animal (urine) starter material or synthesized de novo in a laboratory. To the end user (the human cell) the resulting molecules would be indistinguishable.
“Natural” does not necessarily mean “better” or “safer.” We are now in a world in which “synthetic” is becoming synonymous with “dangerous,” but much modern health care has benefitted from the development of “synthetic” drugs. Conversely, both arsenic and cobra venom qualify as “natural.”
The Women’s Health Initiative (WHI) taught us that there is no substitute for carefully planned, long-term randomized controlled trials looking at real-world endpoints (and not biomarkers). Of course there may be individual patient/prescriber preferences – ethical concerns relating to animal welfare, price, evidence base – but the branding of some formulations as “more natural” is a poor substitute for an evidence base.
Natural estrogens native to humans
The classic trio of human estrogens is formed of 17ß-estradiol (coded E2), estrone (E1), estriol (E3). The “E” shorthand refers to the number of hydroxyl groups on the molecule (Figure 15.1). In the gynecologic field, claims are being made for the potential therapeutic benefits of estetrol (E4), a weak estrogen produced solely by the fetal liver during pregnancy [4].
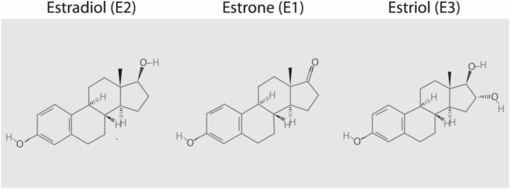
Structure of the three classic human estrogens. (Source: http://pubchem.ncbi.nlm.nih.gov/.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


