References
Country
Year
IBD incidence (per 100,000)
IBD prevalence (per 100,00)
Other comments
Kugathasan et al. [5]
USA
2003
IBD 7.1
N/A
Prospective
CD 4.5
Population based
UC 2.2
Malaty et al. [121]
USA
2010
IBD
Prospective
1.1 (1991–1996)
2.44 (1997–2002)
CD
0.66 (1991–1996)
1.33 (1997–2002)
UC
0.34 (1991–1996)
0.45 (1997–2002)
Benchimol et al. [130]
Canada
2008
IBD
IBD
Health administrative data
9.5 (1994)
42 (1994)
11.4 (2005)
56 (2005)
Barton et al. [7]
UK
1989
IBD 3.9
N/A
Retrospective
CD 2.3
Population based
UC 1.6
Cosgrove et al. [111]
UK
1996
IBD 3.81
CD 16.6
Retrospective
CD 3.1
UC 3.4
UC 0.71
Hassan et al. [118]
UK/Wales
2000
IBD 2.6
N/A
Prospective
CD 1.36
UC 0.75
Armitage et al. [8]
UK/Scotland
2001
IBD 3.8
N/A
Retrospective
CD 2.5
UC 1.3
Ahmed et al. [122]
UK/south Wales
2006
IBD 5.4
N/A
Prospective
CD 3.6
UC 1.5
Sawczenko et al. [123]
UK/Ireland
2001
IBD 5.2
N/A
Prospective
CD 3.1
UC 1.4
Askling et al. [9]
Sweden
1999
IBD 6.9
N/A
Retrospective
CD 3.8
UC 2.1
IC 1.1
Lindberg et al. [116]
Sweden
2000
IBD 4.5
N/A
Prospective
CD 1.3
UC 3.2
Hildebrand et al. [117]
Sweden
2003
IBD 7.4
N/A
Prospective
CD 4.9
UC 2.2
Jakobsen et al. [124]
Denmark
2008
IBD
4.3 (1998–2000)
6.1 (2002–2004)
CD
2.3 (1998–2000)
3.1 (2002–2004)
UC
1.8 (1998–2000)
2.7 (2002–2004)
Stordal et al. [114]
Norway
2004
IBD 4.7
N/A
Prospective
CD 2.7
UC 2.0
Olafsdottir et al. [115]
Norway
1989
IBD 6.8
N/A
Prospective
CD 2.5
UC 4.3
Pozler et al. [12]
Czech Republic
2006
CD 1.25
N/A
Partly retrospective and partly prospective
Kolek et al. [125]
Czech Republic
2004
IBD 2.24
N/A
Retrospective
CD 0.97
UC 1.12
Auvin et al. [15]
France
2005
IBD 3.1
Prospective
CD 2.3
UC 0.8
Bjornsson and Johannsson [112]
Iceland
2000
CD 8.5
N/A
Prospective
Population based
Gottrand et al. [113]
France
1991
IBD 2.6
N/A
Published in French
CD 2.1
UC 0.5
Ott et al. [126]
Germany
2008
IBD 3.96
N/A
Prospective
CD 2.44
UC 1.11
Orel et al. [127]
Slovenia
2009
IBD 4.03
N/A
Retrospective
CD 2.42
UC 1.14
Arin Letamendia et al. [128]
Spain
2008
IBD 2.6
N/A
Prospective
CD 1.74
UC 0.87
Sood et al. [119]
India/Punjab
2003
UC 6.02
UC 44.3
Prospective
Phavichitr et al. [120]
Australia
2003
CD 2.0
N/A
Retrospective
Yap et al. [129]
New Zealand
2008
IBD 2.9
N/A
Prospective
CD 1.9
UC 0.5
Time trends in pediatric IBD: Several studies from Europe have reported a rising incidence of CD and UC. For instance, a Scottish cohort of hospitalized pediatric IBD patients noted a threefold increase in incidence of CD from 1968 to 1983, with essentially no change in incidence of UC [7]. A follow-up study in Scotland aimed at documenting the incidence of pediatric-onset IBD between 1981 and 1995 examined the temporal trends of IBD in Scotland between 1968 and 1995 [8]. The incidence of pediatric-onset CD has continued to rise in Scotland with the prevalence being reported to have increased by 30% since 1983. The authors further concluded that unlike the previous reports, the incidence of childhood-onset UC also increased. Whether this represents a real rise in incidence or merely the inclusion of milder cases remains uncertain. Additional evidence comes from Sweden where the incidence of CD increased from 2.4/100,000 in 1990–1992 to 5.4 between 1996 and 1998. In contrast, the incidence of UC remained stable over the same time period [9, 10] and from Southeastern Norway from 1990–1994 to 2005–2007 [11]. Data from Eastern Europe is similar with a Czech cohort [12] that reported an increased incidence from 0.25/100,000 to 1.25/100,000 in CD patients aged less than 15 years between 1990 and 2001. Although data from North America assessing time trends in pediatric IBD is sparse, a similar increase in CD was reported from a community-based healthcare delivery system in Northern California. The incidence of CD increased from 2.2 to 4.3 between 1996 and 2006, and from 1.8 to 4.9 for UC [13]. An extensive systematic review of published literature from 1950 till 2009 on pediatric onset IBD by Benchimol et al. has shown an overall increased incidence of pediatric IBD by 78% [14]. Sixty percent of the studies showed a statistically significant increase in CD incidence as opposed to only 20% for UC. The increasing incidence of IBD has been contradicted by other studies such as a cohort from Northern France which documented no change in the incidence of CD over a 10-year period [15].
Geographic Trends of Pediatric IBD
Several studies have noted a higher predisposition of IBD in northern latitudes compared to southern regions [15, 16]. This gradient difference is even seen within countries. The study populations with the highest incidence and prevalence rates are reported from the northern latitudes. Although few pediatric studies have assessed this trend, a Scottish report noted a higher incidence of CD in northern Scotland than in the southern regions of the country; this trend was not replicated for UC [17]. However, data are lacking from North America with no prospective multicenter assessment of this observation. A retrospective review of all hospitalized patients in the US (including adults) over a 2-year period (1986–1987) noted higher frequencies of both CD and UC in northern regions and in urban areas [16].
Racial/Ethnicity Trends
Traditionally, IBD is thought to be less prevalent in non-Caucasian populations. This is most probably related to under-representations of non-Caucasians in the study populations/centers. An assessment of the epidemiology of these disorders in non-Caucasians is complicated by a wide range of factors including the absence of population-based registries in ethnically diverse regions, the use of retrospective data, and highly variable clinical presentations which may delay or obscure the diagnosis [18, 19]. Two pediatric studies have also shown comparable incidence and disease characteristics in African Americans compared with Caucasian populations in two different geographical locations of the US—Wisconsin [5] and Georgia [20]. These studies demonstrate differing proportions of African Americans afflicted with IBD and therefore suggest IBD in African Americans is not rare among pediatric populations. Another report of African American patients from the Pediatric IBD consortium, comprising six academic and community pediatric gastroenterology practices, has described older ages at diagnosis of Crohn disease in African American patients vs. non-African American patients [21].
Jewish ancestry is thought to confer increased susceptibility to IBD. One study showed a lifetime risk of developing IBD in offspring of an affected non-Jewish parent to be 5 and 2% for CD and UC, respectively [22]. The risk increased to 8 and 5% if the affected parent was Jewish. In addition, if both parents are affected, the lifetime risk of developing IBD increases to 33% by age 28.
Hygiene Hypothesis and Other Epidemiological Observation in IBD
A remarkable change in the types of diseases affecting humans has occurred in the developed world over the last century [23]. The most common illnesses responsible for the majority of morbidity and mortality have shifted from infectious diseases to chronic inflammatory diseases and cancer. This was initially noted in Northern Europe and North America (Fig. 5.1) but, since the end of World War II, this phenomenon has been observed in other parts of the world, such as Japan, Eastern Europe, and some South American countries. The emergence of chronic autoimmune disorders and chronic inflammatory diseases (including IBD) throughout the world, has been closely linked to social and economical progress. Keeping with this trend, the emergence of IBD has most recently been observed in the Asian Pacific Region (Fig. 5.1) [24]. The “hygiene hypothesis” has been proposed as the probable underlying reason for the switch from infectious to chronic inflammatory diseases such as IBD, and postulates that there has been a fundamental lifestyle change from one associated with high microbial exposure to one with low microbial exposure [25]. The lack of microbial antigens in infancy and childhood therefore leads to a less educated and weaker immune system unequipped to properly handle new challenges later on in life. This lead to generating an ineffective immune response that is prolonged and inappropriate because it is powerless to eliminate the offending agent. Many environmental modifications have been ascribed to the hygiene hypothesis, including better and bigger housing, safe food and water, improved hygiene and sanitation, vaccines, widespread use of antibiotics leading to lack of parasites, fewer infections, and better nutrition [26]. While contributing to the progressive decline of infectious diseases, these changes may have simultaneously contributed to a surge in allergic and immunologic diseases.
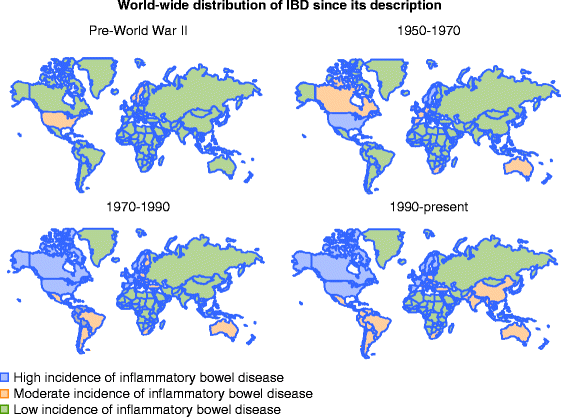

Fig. 5.1
Worldwide distribution of inflammatory bowel disease (IBD) since its description
Environmental Risk Factors (Fig. 5.2)
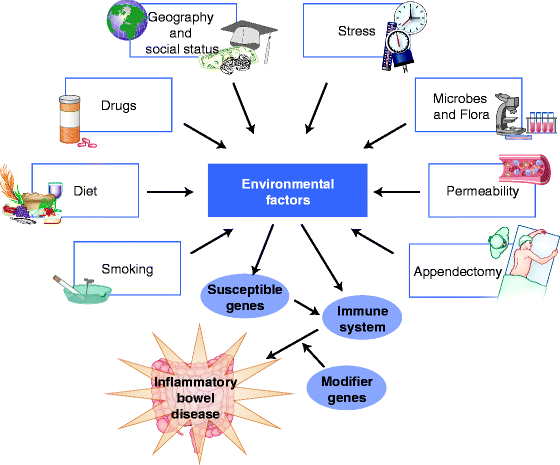
Fig. 5.2
Environmental factors implicated in IBD pathogenesis
Smoking
The most indisputable example of the influence of the environment on IBD is tobacco use, specifically, cigarette smoking. Smoking has a strikingly opposite effect on CD as compared to UC, supporting the notion that distinct mechanisms underlie the pathogenesis of each of these forms of IBD [27, 28]. Notably, UC patients are frequently nonsmokers, and furthermore, cessation of smoking increases the risk of developing UC. In fact, nicotine patches are currently being used to treat UC, supporting its protective role in this disease.
The role of passive smoking, particularly in children, as either a risk factor or protective factor for CD and/or UC is still a matter of controversy, with none of the studies having quantitatively assessed passive smoke exposure. The mechanisms underlying the differential effect of smoking in CD or UC are also obscure. However, smoking has been demonstrated to affect both systemic and mucosal immunity, as well as alter a wide range of both innate and adaptive immune functions [29]. For instance, smoking alters the ratio of T-helper to T-suppressor cells, reduces T cell proliferation, modulates apoptosis, and significantly decreases serum and mucosal immunoglobulin levels. In animal models, smoking reduces mucosal cytokine production and promotes adhesion of leukocytes to endothelial cells. Furthermore, it enhances small bowel permeability and colonic mucus production. Interestingly, transdermal nicotine shows some beneficial effect in patients with mild-to-moderate UC. On the other hand, nicotine may be detrimental in CD by contributing to the hypercoagulability state present in this condition. Taken together, these divergent effects of smoking in human IBD indicate a complex interaction between smoking and IBD [30]. In childhood IBD, a concerted effect should be made to study smoking exposure and the risks of IBD development, progression, as well as its interaction with an individual’s genes in determining the eventual outcome.
Microbial Factors; Specific Infectious Agents
The search for specific infectious organisms as a cause of IBD has remained very attractive. The history of IBD is dotted by cyclic reports on the isolation of specific infectious agents thought to be responsible for CD or UC. Unfortunately, none of these initial reports have ever been reproduced. Several microorganisms have been proposed as having potential etiologic roles, such as Listeria monocytogenes, Chlamydia trachomatis, Escherichia coli, Cytomegalovirus, Saccharomyces cerevisiae, and others. Among those, the role of Mycobacterium avium paratuberculosis (MAP) in CD has been the center of major controversy. This bacterium is the causative agent of Johne’s disease, a chronic granulomatous ileitis in ruminants that closely resembles CD. MAP was initially isolated from a few CD tissues [31], but follow-up studies that tried to culture M. paratuberculosis looked for specific DNA sequences in intestinal samples, or measured serum antibodies against M. paratuberculosis yielding conflicting or inconclusive results. In addition, controlled trials have repeatedly failed to show a therapeutic effect of antituberculous therapy in CD patients [32]. Recently, however, there has been a renewed interest in MAP as a cause of CD following the isolation of MAP by PCR in milk sold in supermarkets in California and Wisconsin [33].
In addition to bacteria, a few have proposed a viral etiology as the cause of IBD and specifically for CD. The finding of paramyxovirus-like particles in CD endothelial granulomas has led to the suggestion that CD is a chronic vasculitis caused by the persistence of the measles virus in the mucosa [34]. In support of this hypothesis, an association between perinatal measles and predisposition to CD was also advanced based on some epidemiological and serologic data [35]. Despite these preliminary findings none were confirmed by later investigations [36].
Importantly, the progressive decline of measles virus infection in the last decades with the concomitant rise of CD during the same period of time, speaks largely against an etiologic role of measles in CD. The hypothesis that the measles vaccination, rather than measles infection, might be a risk factor for CD, has also been raised but again subsequent studies failed to confirm any association [37].
Microbial Factors; Intestinal Commensal Flora
Over the past decade, there has been an exponential increase in interest about commensal bacteria as etiological agents of IBD. Based on fairly solid data, a substantial body of evidence has accumulated suggesting that the normal enteric flora plays a key role in the development of IBD [38]. In addition, the following clinical observations support this hypothesis: (1) that the beneficial effect of antibiotics in the treatment of CD, and to a lesser extent UC, has been appreciated for years; (2) diversion of the fecal stream from inflamed bowel loops has been known to induce symptomatic improvement in CD patients, while relapse often occurs upon restoration of intestinal continuity; and (3) pouchitis, a chronic inflammation of a surgically constructed ileo-anal pouch, develops in a considerable proportion of UC patients after proctocolectomy, and is associated with a dysbiosis caused by the contact of the once near sterile small bowel mucosa with a rich colon-like flora repopulating the pouch after surgery [39]. Furthermore, the more recent demonstration that probiotics (primarily lactic acid bacteria), defined as live microbial feeds, beneficially affect the host by modulating gut microbial balance, and improves both human IBD and experimental colitis, adds an important dimension to the role of gut flora in IBD. In addition, much larger numbers and concentrations of bacteria make up the biofilm covering the intestinal epithelium of IBD patients compared to the epithelium of healthy subjects [40]; and loss of immune tolerance against the autologous enteric aerobic and anaerobic flora has been reported [41]. Finally, and probably the most convincingly, is that, in the majority of animal models of IBD, intestinal inflammation fail to develop when they are kept in a germ-free environment [42]. Why there is an abnormal response to normal endogenous gut bacteria in IBD is not clear, but the recent discovery that CD is genetically associated with mutations of the NOD2 gene, whose products are bacteria-recognizing proteins, points to a link between gut inflammation and abnormal bacterial sensing [43]. Many other identified genes are also actively involved in regulating the interface between the intestinal epithelium and gut microorganisms. One model suggests a 3-step scenario in which bacteria penetrate the epithelial barrier, provoking a weak inflammatory response with impaired clearance in certain persons, which in turn causes chronic inflammation, culminating in IBD [44]. The relative importance of commensal organisms vs. intestinal pathogens in the initiation and/or relapse of IBD remains under debate.
Appendectomy
Protective effects of appendectomy upon UC have been well established in a number of adult and pediatric studies. A meta-analysis of 17 case-control studies showed a 69% reduction in risk of subsequent development of UC in cases with appendectomy [45, 46]. This negative association is more pronounced in pediatric onset UC. Duggan et al. [47] also noted a protective effect of appendectomy on development of UC, especially if performed during childhood. On the other hand, risk for CD is reported to be increased after appendectomy [48]. The reasons for these observed differences remain ill defined with altered mucosal immunity in response to appendectomy as one of the proposed hypothesis.
Breast Feeding
Breast feeding is thought to provide protection against infections and minimizes allergic triggers to a developing gut. It has also been suggested to provide long-term beneficial effects like improved cognitive functioning, prevention of chronic diseases like type I diabetes and celiac disease, lowering of blood pressure, and an improved cholesterol profile [49]. The role of breast feeding (or lack there of) has been reported to be associated with development of Crohn disease in a few pediatric studies. A meta-analysis by Klement et al. suggested protective effects of breast feeding on later development of IBD [50]. This has been contradicted by a population-based case-control study which reported an increased risk of CD among breast-fed infants [51]. They also reported an increased risk of CD with eczema and BCG vaccines, but a protective effect of drinking tap water. Meanwhile, the risk of developing UC increased with family history of IBD, disease during pregnancy, and bedroom sharing, but appendectomy conferred a protective effect.
Dietary Factors
The gastrointestinal tract is constantly being exposed to dietary antigens, and hence, this exposure has generated a number of observations relating to the potential role of different dietary components in the pathogenesis of IBD. Given the location of IBD, this relationship between components of the diet and disease has been long considered, and immunologic mechanisms have been postulated to link food antigens with the development of intestinal inflammation. However, this logical and appealing explanation is far from proven. In addition, only a few unpersuasive studies provide indirect evidence only, of a possible cause-and-effect relationship between specific dietary factors and IBD. Most of these case-control studies are limited by methodological problems including diet recall and role of preillness diet vs. diet selection post diagnosis. Contrary to these case-control studies, a population-based study by Baron et al. [51] failed to identify any specific dietary factors as potential risk factors for development of IBD. However, a questionnaire-based, population case-control study from Manitoba, Canada reported reduced ingestion of pork and un-pasteurized milk as significantly associated with IBD compared to controls, while no difference was seen with beef or chicken meat ingestion, although the frequency of chicken intake was noted to be higher in patients with CD as compared to patients with UC [52]. Ingestion of refined sugars has also been reported to be associated with CD [52, 53]. Gilat et al. showed higher consumption of whole wheat bread and lower consumption of oat meal cereals in CD patients [54]. Lastly, there is some evidence supporting the benefits of elemental and polymeric liquid diets as primary or adjuvant therapies for CD, although in some studies this nutritional approach was less effective than conventional therapies, such as corticosteroids [55].
Drugs
Oral contraceptives (OCP) and nonsteroidal anti-inflammatory drugs (NSAIDs) are the two main classes of drugs that have been intensively studied for a possible epidemiological or causal relationship with IBD. Although there is no direct evidence for a causative relationship, the relative risk of CD in women taking OCPs is about twice that of controls [56]. In contrast, prevalence of usage of OCP in females in a case-control study from Canada [52] did not differ in patients with IBD compared with controls; however, CD patients were more likely to use OCPs than controls and patients with IBD tended to start OCPs at an earlier age than the controls. Inverse causality has also been implicated in OCP use in patients with abdominal symptoms since they are routinely used to treat menstrual associated complaints. The situation is less ambiguous in the case of NSAIDs, because their use is clearly associated with a higher risk of IBD. Primarily, IBD patients in clinical remission can relapse upon NSAIDs administration. In fact, IL10 knockout mice spontaneously develop colitis when given NSAIDs and exhibit a far more rapid and severe form of colonic inflammation associated with blockade of protective prostaglandins and altered mucosal immune reactivity [57], suggesting a potential mechanism by which NSAIDs may worsen IBD. However, NSAIDs and OCPs are less likely to be an important contributing factor in childhood onset IBD.
Another medication that has been frequently associated with IBD is Isotretenoin. The first case report of this association was reported in 1986 by Brodin [58]. Other cases then followed, leading to caution in using this medication as advertised by the drug manufacturer [59, 60]. However a number of careful and systematic reviews have raised question about this association and no evidence currently exists to convincingly support this [61, 62].
Risk Prediction of Disease Progression
The risk of surgery in CD patients has been studied in children and is reported to vary between 5% at 1 year after diagnosis and 36% at 5 years after diagnosis depending upon single center or multicenter studies [63, 64]. The two multicenter studies [63, 65] have reported a 5-year risk of 17% and 10-year risk of 28%. Clinical features that predict complicating disease (defined as stricturing or internal penetrating disease) in patients with CD include older age at onset, poor growth, small bowel disease, abscess, female gender, worsening disease activity as measured by physician global assessment (PGA), and presence of strictures and fistulae. Biochemical parameters predicting risk of surgery in children include leukocytosis and hypoalbuminemia. One caution that we have to exercise in these studies is the variability in defining disease characteristics. some of these studies that have used descriptive disease location, are retrospective, and others used Vienna classification [66]. As noted by Vernier-Massouille et al. [67], over time the inflammatory phenotype of CD tends to decrease, converging with the increasing prevalence of the stricturing phenotype. A newer, more consistent Paris classification [68] may help address some of these concerns and help in unifying these different phenotypes.
A number of serologic immune markers along with genetic polymorphisms have been associated with CD and UC. The sensitivity and specificity of these immune markers is variable and ranges from 65 to 80% for sensitivity and 92 to 94% for specificity [69–71]. These, therefore, are not useful as screening tests. Their utility, however, has been studied as markers for predicting disease complications. For example, it has been shown that ASCA is associated with small bowel disease location [72], risk of surgery [73], and complicated disease course [74]. High levels of pANCA and anti-CBir1 in UC patients are predictive of development of pouchitis after proctocolectomy [75]. However these markers are not specific for IBD and have been seen in non-IBD diseases like autoimmune hepatitis, primary sclerosing cholangitis [76], ankylosing spondylitis, rheumatoid arthritis [77], diabetes [78], cystic fibrosis [79], and infections like tuberculosis [80].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


