ETIOLOGY, PREVENTION, AND MOLECULAR BIOLOGY
LOUISE A. BRINTON  VIKRANT V. SAHASRABUDDHE
VIKRANT V. SAHASRABUDDHE  BRITTON TRABERT
BRITTON TRABERT
Disease-oriented texts often include a chapter on epidemiology or etiology, which is considered perfunctory if the book is used by therapists whose daily practice is rarely influenced by these considerations. This is not the case for physicians who treat patients with gynecologic cancers, because these clinicians have frequent opportunities to interpret epidemiologic findings and make observations of etiologic importance. Moreover, public health measures based on epidemiologic findings influence gynecologic practice perhaps more than any other clinical discipline. In particular, epidemiologic data are critical for the prevention and early diagnosis of cervical and uterine cancers.
From the observation 150 years ago of the rarity of cervical cancer in nuns to the most recent follow-up studies of type-specific human papillomavirus infection, determining the cause, natural history, and prevention of this disease has focused on sexual practices and suspect infectious agents. Screening interventions based on natural history studies have fundamentally altered the usual presentation of this disease, and as more information about preceding infectious processes becomes available, even more radical changes in presentation and management are likely.
The probable estrogenic cause of endometrial cancer was proposed by etiologically oriented gynecologists decades before its demonstration by epidemiologists. Unfortunately, this did not prevent the largest epidemic of iatrogenic cancer in recorded history (i.e., endometrial cancer caused by unopposed estrogen therapy). The resurgent interest in menopausal hormone therapy, effects of progestins added to this regimen, and associated risk-benefit questions are certain to link the epidemiologist and the gynecologist for the foreseeable future. The iatrogenic chemoprevention of endometrial and ovarian cancers through oral contraception has similarly thrust the two disciplines together around issues ranging from basic biology to risk-benefit assessments.
The rich tradition of the mingling of epidemiology and gynecologic oncology has led to better opportunities for prevention, screening, and gaining insights into basic mechanisms of disease than for any other subspecialty concerned with cancer. This chapter is written with the aim of clarifying how epidemiology is an integral part of the effort to reduce the morbidity and mortality from gynecologic cancers in women.
In this chapter, we review results from a number of epidemiologic investigations, mainly observational studies, including cohort and case-control studies. There have been a number of large cohort studies focused on women, such as the Nurses’ Health Study and the Million Women Study, and these have provided much useful information regarding etiologic factors for gynecologic cancers. However, these investigations, which collect baseline information and then follow subjects forward in time to capture information on incident cancers, are often limited in terms of the exposure information that is captured. They also are not usually useful for exploring risk factors for rare malignancies, and thus, we also depend on results from case-control investigations to clarify risks. Although these studies more often raise concerns regarding the possible role of selection and recall biases because of the retrospective nature of the information gathered from women with certain cancers and nondiseased comparison subjects, they oftentimes collect more exposure information that can be informative in terms of controlling for the influence of confounding factors and assessing interactions between risk factors (effect modification). The issue of control for confounding (i.e., disentangling effects of correlated variables in terms of identifying the independence of effects) is an important issue for both case-control and cohort studies.
In the studies reviewed below, various measures of risk are discussed, including relative risks (RR) from cohort studies and odds ratios (OR) from case-control studies. The RR from a cohort study represents the incidence of disease in women exposed to a factor compared to the incidence in unexposed women. The OR from a case-control study is an approximation of the RR determined by comparing the odds of developing the disease based on an exposure compared to the odds based on lack of the exposure. There are uncertainties with both these measures, which are influenced by the prevalence of the exposure, the incidence of the disease, the numbers of cases included in a study, and the amount of random variability inherent in the data-collection process. In an attempt to quantify the random error that underlies the risk estimate, the RRs or ORs are usually accompanied by derived 95% confidence intervals (CI). The 95% CI means that if the data collection and analysis were replicated an infinite number of times, the CI would include the true risk estimate 95% of the time. Significantly elevated or decreased risks are indicated, respectively, if the lower confidence limit exceeds 1.0 or the upper confidence limit is less than 1.0. Thus significance levels are comparable, although not precisely equivalent, to p < 0.05, which is commonly used as a standard level of acceptance of a risk that is interpreted as importantly elevated or decreased.
UTERINE CORPUS CANCER
Demographic Patterns
Cancer of the uterine corpus (hereafter referred to as uterine cancer) is the most common invasive gynecologic cancer and the fourth most frequently diagnosed cancer among U.S. women today. One in 40 U.S. women will develop uterine cancer during her lifetime, and it is estimated that there were approximately 46,470 diagnoses during 2011 (1). The average annual age-adjusted (2000 U.S. standard) incidence from the Surveillance, Epidemiology and End Results (SEER) program, a cancer reporting system involving approximately 28% of U.S. residents, was 23.9 per 100,000 women for 2004–2008; the corresponding age-adjusted mortality rate was 4.2 per 100,000 women, reflecting the relatively good prognosis for this cancer (2). The 5-year survival rate is approximately 81.8%. It is estimated that approximately 8,010 women will die from uterine cancer during 2012 (1).
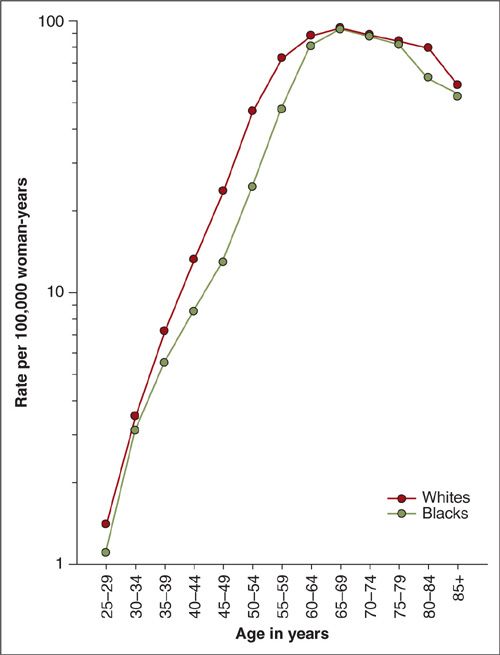
Uterine cancer rates are generally highest in North America and Northern Europe, intermediate in Southern Europe, temperate in South America, and low in Southern and Eastern Asia (including Japan) and in most of Africa (except southern Africa) (3) (Fig. 1.1). The disease is rare before the age of 45 years, but the risk rises sharply among women in their late 40s to middle 60s (Fig. 1.2). The age-adjusted incidence for Whites is approximately twice the incidence for non-Whites (Fig. 1.3). Reasons for the discrepancy remain largely undefined. Within the last several decades in the U.S., a dramatic change in the incidence pattern for uterine cancer has occurred, characterized by a marked increase that peaked around 1975 (4). Considerable evidence has linked this rise and fall with the widespread use of unopposed estrogen therapy in the late 1960s and early 1970s. Mortality rates, albeit considerably lower, have generally mirrored incidence rates (Fig. 1.3).
Reproductive Risk Factors
Nulliparity is a recognized risk factor for uterine cancer. Most studies demonstrate a two- to threefold higher risk for nulliparous women than for parous women. The association of uterine cancer with nulliparity has been suggested to reflect prolonged periods of infertility. The hypothesis that infertility is a risk factor for uterine cancer is supported by studies showing higher risks for married nulliparous women than for unmarried women. Several studies have found that infertile women experience a three- to eightfold increase in risk (5,6). Mechanisms that may mediate the risk associated with infertility include anovulatory menstrual cycles (i.e., prolonged exposure to estrogens without sufficient progesterone), high serum levels of androstenedione (i.e., excess androstenedione is available for conversion to estrone), and the absence of monthly sloughing of the endometrial lining (i.e., residual tissue may become hyperplastic). In addition, nulliparity has been associated with lower levels of serum sex-hormone–binding globulin (SHBG), leading to increased bioavailable estrogen (7).
It has been established for many years that the risk of uterine cancer decreases with increasing parity, especially among premenopausal women (8,9). More recent attention has focused on characteristics of ages at which these births occurred. Several investigators have found decreased risks with either older ages at or shorter intervals since a last birth, and have suggested that this might reflect a protective effect of mechanical clearance of initiated cells (10,11).
An understanding of the effects of infertility on cancer risk must also consider relationships according to different methods of birth control, including oral contraceptives (discussed later in this chapter). However, it is also of interest that a number of investigations have noted reductions in risk among users of intrauterine devices, as discussed in a recent meta-analysis (12). The mechanisms involved with this apparent protective effect have not been elaborated, although it is possible that the devices may affect risk by causing structural or biochemical changes that alter the sensitivity of the endometrium to circulating hormones.
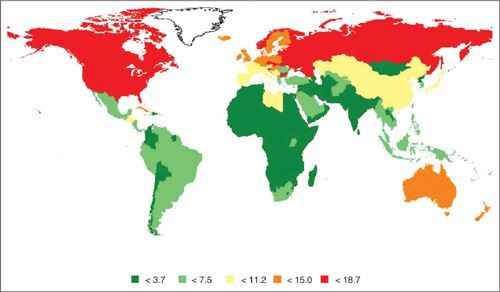
FIGURE 1.1. International incidence for uterine cancer (per 100,000 woman years) age-standardized to the world population, 2008.
Source: Ferlay J, Shin JR, Bray F, et al. GLOBOCON 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010; Available from: http://globocan.iarc.fr, acced on 23 March 2012.
FIGURE 1.2. Age-specific uterine cancer incidence rates by race, U.S. SEER-17, 2000–2008.
An additional area of interest is the effect of exposure to fertility drugs, given several studies that have shown that users of ovulation-inducing drugs are at an increased risk of developing uterine cancers (13,14). Although this observation requires replication, it is of interest given the structural similarity of clomiphene and tamoxifen, which has been extensively linked with the occurrence of uterine cancers (discussed in more detail below).
The relationship of risk to breast-feeding remains controversial. Although some studies suggest that prolonged lactation may offer protection (15), this has not been noted in all investigations (11).
Menstrual Risk Factors
Early ages at menarche have generally been related to an elevated risk for uterine cancer in many studies. A recent report from a large multicenter prospective cohort reported a 30% reduction in risk with late age at menarche and an inverse dose-response trend (11). Stronger effects of ages at menarche may prevail for younger women (11). The extent to which these relationships reflect increased exposure to ovarian hormones or other correlates of early menarche (e.g., increased body weight) is unresolved.
Most studies have indicated that age at menopause is directly related to the risk of developing uterine cancer. Approximately 70% of all women diagnosed with uterine cancer are postmenopausal. Most studies support the estimate that there is about a twofold risk associated with natural menopause after the age of 52 years compared to before the age of 49 years (16). It has been hypothesized that the effect of late age at menopause on risk may reflect prolonged exposure of the uterus to estrogen stimulation in the presence of anovulatory (progesterone-deficient) cycles. The interrelationships among menstrual factors, age, and weight are complex, and the biologic mechanisms of these variables operating in the pathogenesis of uterine cancer are subject to substantial speculation.
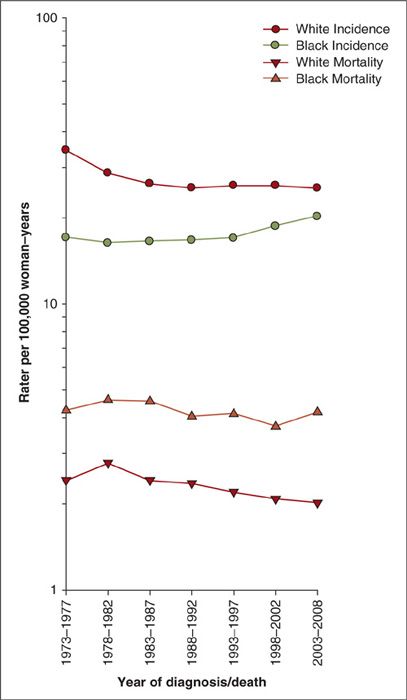
FIGURE 1.3. Uterine cancer incidence and mortality trends among US women, SEER-17, 1973–2008.
Exogenous Hormones
Oral Contraceptives
The use of combination oral contraceptives may reduce the risk of uterine cancer by 40% to 60%, and long-term use may decrease the risk further (11,17). One meta-analysis noted that the decreased risk persisted more than 20 years after ceasing use (18). In several studies, the greatest reduction in risk was associated with high-progestin-dose pills, although recent findings indicate that this may be true only among obese women (19).
Menopausal Hormones
It is well established that unopposed estrogens are associated with a 2- to 12-fold elevation in uterine cancer risk (20). In most investigations, the increased risk does not become apparent until the drugs have been used for at least 2 to 3 years, and longer use of estrogens is generally associated with higher risk. The highest RRs have been observed after 10 years of use (up to 20-fold), although it is unclear whether risk increases after 15 years. Most but not all studies have found that cessation of use is associated with a relatively rapid decrease in risk, although a number of studies have found significantly elevated risks to persist 10 or more years after last usage. Although higher doses of estrogen are associated with the greatest elevations in risk, one study showed that even 0.3 mg of unopposed equine estrogen can result in a significant increase in risk (21).
This large body of evidence linking estrogen use to increases in the risk of uterine cancers has led to estrogens being prescribed in conjunction with progestins among women who have not had a hysterectomy. Progesterone has been shown to cause regression of endometrial hyperplasia, the presumed precursor of uterine cancers (22). The large Women’s Health Initiative (WHI) clinical trial showed that women assigned to 0.625 mg of conjugated equine estrogen plus 2.5 mg of medroxyprogesterone acetate daily had a lower hazard ratio (0.81, 95% CI: 0.48–1.36) than those assigned to placebo, but this risk was based on relatively small numbers of endometrial cancers and short follow-up (23). Similar observational results derive from the Million Women Study in the United Kingdom, where usage of continuous combined therapy resulted in a relative risk of 0.71 (95% CI: 0.56–0.90) (20).
Although a number of studies indicate that the excess risk of uterine cancer associated with estrogens can be significantly reduced if progestins are given for at least 10 days each month (24,25), some studies have shown that subjects prescribed progestins for less than 10 days per month (sequential users) experience some increase in risk, with only a slight reduction compared to estrogen-only users (26,27). The sharp contrast between the effects of <10 and ≥10 days of progestin use has led to the suggestion that the extent of uterine sloughing or of “terminal” differentiation at the completion of the progestin phase may play a critical role in determining risk. It remains questionable whether 10 days of progestin administration per month is sufficient for complete protection, particularly for long-term users (24). Few studies have had large numbers of long-term sequential users, but there is some evidence that this pattern of usage may result in some persistence of risk (28).
Studies have shown that the effects of hormonal therapy (both unopposed estrogens as well as combination therapy) may vary by user characteristics, most notably by a woman’s body mass. A number of studies have shown that the adverse effects of unopposed estrogens were greatest in nonobese women and that the beneficial effects of combined therapy were greatest in obese women (20,28,29).
Most data regarding effects of hormones derive from studies on users of pills. Unresolved is whether the use of estrogen patches, creams, or injections can affect risk; given relationships of risk with even low-dose estrogens, it is plausible that these regimens may confer some increase in risk.
Tamoxifen
A number of clinical trials and one population-based case-control study have demonstrated an increased risk of uterine cancer among tamoxifen-treated breast cancer patients (30,32). This is consistent with tamoxifen’s estrogenic effects on the endometrium. Elevated risks have been observed primarily among women receiving high cumulative doses of therapy, usually in the range of 15 g or greater. The risk for more high-risk endometrial cancer histologies may be especially elevated (33).
Anthropometry and Physical Activity
Obesity
Obesity is a well-recognized risk factor for uterine cancer and may account for up to 25% of cases (34). Very heavy women appear to have exceptionally high risks (29). Although studies have demonstrated significant positive trends of uterine cancer with both weight and various measures of obesity, including BMI (weight / height2), height has not been consistently associated with risk. Obesity appears to affect both premenopausal and postmenopausal uterine cancer.
Although initial studies hypothesized that adolescent and long-standing obesity may be more important than adult weight, recent studies support that contemporary weight and weight gain during adulthood are the most important predictors of uterine cancer risk (29,35). Relationships with obesity may be stronger for postmenopausal disease and among women not exposed to exogenous hormones (29,36).
Recent interest has focused on determining whether the distribution of body fat predicts uterine cancer risk. A number of studies have shown that central obesity may have an effect independent of overall body size (29), although not all studies confirm this relationship.
Physical Activity
Recent investigations have focused on the role of physical activity in the etiology of uterine cancer. A potential relationship is biologically appealing given that physical activity can result in changes in the menstrual cycle, body fat distribution, and levels of endogenous hormones. Accumulating evidence suggests a protective effect of physical activity on uterine cancer risk independent of relationships with body weight. A recent meta-analysis of prospective studies demonstrated a decreased risk of uterine cancer with moderate-to-vigorous physical activity independent of relationships with known potential confounders, namely obesity, menopausal hormone therapy use, and parity (37). Generally, studies that have evaluated occupational and recreational physical activity have reported decreased risks.
Inadequate physical activity has been evaluated as a potential risk factor for uterine cancer; specifically sedentary behavior, often measured as sitting time, has been associated with increased risk of uterine cancer (37,38). It is still not clear, however, from the existing studies if the association is independent of moderate-to-vigorous physical activity or body weight (37). Indirect support for a link between sedentary behavior and uterine cancer risk comes from occupational studies, in which the increased risk among women with sedentary jobs is very clear.
Cigarette Smoking
A reduced risk of uterine cancer among smokers has been reported, with current smokers having approximately half the risk of nonsmokers (39–41). Cigarette smoking has been linked to an earlier age at natural menopause in some populations and to reduced levels of endogenous estrogens. Reduced risks may be more pronounced in parous or obese patients (42).
At present, biologic mechanisms underlying the inverse relationship of smoking to uterine cancer risk remain elusive. Alterations in endogenous hormones or metabolites are likely involved. In one report (43), the inverse association of smoking with uterine cancer risk appeared to be more strongly related to higher serum androstenedione levels than to lower serum estrogen levels, except perhaps among overweight women.
Dietary Factors
Despite the fact that obesity has been consistently related to uterine cancer, the role of dietary factors remains controversial. Geographic differences in disease rates (i.e., high rates in Western and low rates in Eastern societies) suggest that nutrition has a role, especially the high content of animal fat in Western diets. Armstrong and Doll (44) demonstrated a strong correlation between a country’s total fat intake and uterine cancer incidence.
Dietary Fat
Although early studies speculated that dietary fat intake (particularly animal fat) might play a role in the etiology of uterine cancers, results from more recent studies have provided inconsistent results (45–47
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree