The mortality from neonatal infections is considerably lower in resource-rich countries. Intrapartum antibiotic use to prevent group B streptococcus (GBS) infection has reduced mortality significantly in countries with a high incidence.2-4
Newborn babies have rates of infection as high as children and adults whose immunity is compromised for almost any other reason, including most oncology patients and the elderly. Although newborn babies, and particularly pre-term newborns, are immunocompromised, additional factors contribute to the high rates of neonatal infection and will be considered in Section 2.2.
Knowledge of the incidence of neonatal infections is important for planning preventive and intervention strategies and for comparisons within and between countries, which can help inform clinical practice and help assess the quality of care. However, such comparisons are not necessarily straightforward.
In developing countries, most deliveries occur in the home, so hospital-based studies of incidence and aetiology may give misleading or inaccurate results. Infections are usually diagnosed clinically without cultures, and deaths from infection are frequently under-reported. Community studies report rates of clinical neonatal sepsis ranging from 49 per 1000 live births in babies >24 hours old in Guatemala to 170 per 1000 live births in rural India.2 The reported rate of blood culture-confirmed cases is far lower: a minimum of 5.5 per 1000 live births in a rural hospital in Kenya,5 a highly uncertain figure because of incomplete sampling. Infection-specific mortality rates reported in 32 studies varied from 2.7 per 1000 live births in South Africa to 38.6 per 1000 in Somalia.2
In industrialized Western countries where most de liveries occur in hospital, hospital-based studies of incidence are more representative.
The reported incidence of neonatal infection depends on how neonatal infection is defined and reported. Definitions may vary considerably. Examples include how contaminants in blood cultures and cerebrospinal fluid (CSF) are defined; whether or not contaminants are excluded; whether or not clinical sepsis with evidence of raised inflammatory markers is accepted as being infection; and whether infections are confined to positive cultures of blood and/or CSF or also include positive cultures from normally sterile sites, such as urine, bone, joint fluid or pulmonary fluid.
2.1.1 Early-onset infection
It is conventional to divide the reporting of neonatal infections into early- and late-onset infections. Early infections are presumed to be due to organisms acquired from the mother shortly before (e.g. Listeria) or at the time of birth (e.g. GBS) whereas late infections are primarily caused by environmental organisms, acquired nosocomially (i.e. in hospital) or in the community. However, ‘early onset’ has been defined as anything from the first 2 days to the first 7 days after birth. Furthermore, environmental organisms may grow from blood cultures in the first 48 hours after birth, while the classic early-onset organisms like GBS and Listeria can cause late- as well as early-onset sepsis. Methicillin-resistant Staphylococcus aureus (MRSA), originally confined to hospitals or patients associated with hospitals, is a common community-acquired pathogen in many countries and can colonize pregnant women and cause both early- and late-onset neonatal infections.
There are two major considerations in defining early-onset infection, clinical and epidemiologic.
Figure 2.2 Timing of neonatal infection by organism. Adapted from Reference 6. CoNS, Coagulase-negative staphylococci; Other GNB, Other Gram-negative bacilli; Gp B Strep, Group B streptococci.
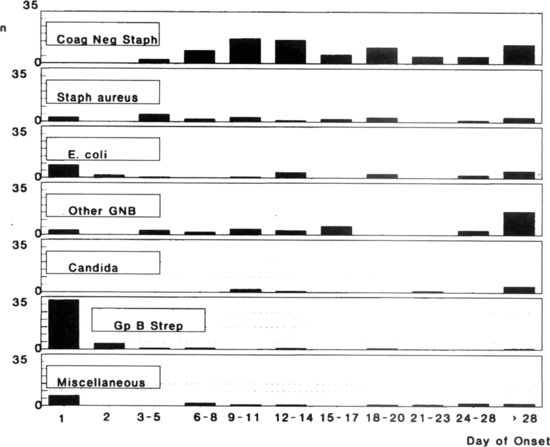
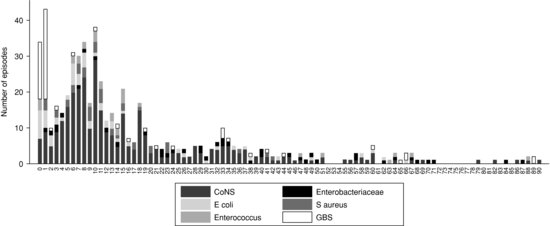
Figure 2.3 Pathogens according to the day of onset of infection, UK 2006–2008. Reprinted from Reference 7 with permission. CoNS, Coagulase-negative staphylococci; GBS, Group B streptococci.
2.1.2 Late-onset infection
Late-onset neonatal infection can be caused by organisms associated with neonatal intensive care and acquired nosocomially (nosocomos means hospital in Greek), for example, CoNS and water-loving Gram-negative bacilli; by maternally acquired organisms that colonize the baby at birth and invade later, for example, GBS and Listeria; or by community organisms, including respiratory, gastrointestinal and Salmonella and skin organisms.13
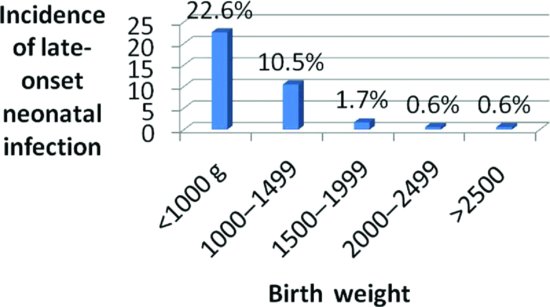
In industrialized Western countries, the major burden of late-onset neonatal sepsis is in babies in neonatal intensive care units (NICU). The incidence of late sepsis is inversely related to gestational age and birth weight14 (see Figure 2.4). Incidence can also be reported in terms of the proportion of babies admitted to NICU who develop sepsis, usually around 2–5%, depending on the NICU population.7-14 In one study, annual rates of late sepsis ranged from 2.4% to 4.5% of NICU admissions in different NICUs, a difference which was statistically significant, but no longer significant after stratifying for birth weight.14
Rates of late-onset neonatal sepsis in industrialized countries may be reported as the proportion of babies admitted to neonatal units who develop sepsis or the proportion of all live born babies who develop sepsis, if these data are known. However, even after allowing for birth weight, the comparisons may be confounded by population variation. The risk of infection increases with the duration of neonatal unit stay, particularly if intensive care is needed. Stratification by birth weight allows for this, because low birth-weight infants stay longer. However, other more mature infants may require intensive care, for example, those needing gastrointestinal surgery.
In industrialized countries, the reported rate of late-onset neonatal infection in neonatal units is most useful when definitions of sepsis are standardized. Even then, comparisons between hospitals are only truly valid when variations in patient populations have been taken into account.15,16
A refinement of crude comparisons of overall rates of late sepsis is to compare specific infections that are felt to reflect good hygiene in intensive care. An example is the use of rates of central line-associated bacteraemia (CLAB), also called central line-associated blood stream infections (CLABSI).15 A CLAB is defined as a bacteraemic infection caused by an organism like CoNS in a baby with a central line in situ and no other explanation for the infection. The rate can be reported as the number of CLAB’s per 1000 line days, which makes allowance for varying duration of exposure (see Section 20.2.1).
Widespread use of artificial surfactant in resource-rich countries has permitted early extubation of almost all pre-term infants, who are then managed with nasal continuous positive airways pressure (CPAP). Infants on CPAP are at low risk of ventilator-associated pneumonia (VAP), so VAP is no longer a useful measure of nosocomial infection in newborns (see Chapter 8).
In developing countries, the reliability of reported infection rates depends on factors such as definitions, case ascertainment, population selection, whether or not babies are cultured and the reliability of the microbiology laboratory.2 A review of 32 studies from developing countries found considerable study heterogeneity, making comparisons difficult.2 Many studies did not distinguish early from late sepsis. Clearly, more accurate data based on standardized methods are needed.
2.1.2.1 Organisms causing late sepsis
In most Western countries, CoNS now cause just over 50% of all late-onset neonatal infections, even when likely contaminants are excluded. Most CoNS infections occur in low birth-weight infants and are central line-associated.6-15 The major pathogens causing the remainder of late-onset infections are Gram-negative enteric bacilli (Enterobacteriaceae) and S. aureus (methicillin-sensitive or MRSA). Other important but rarer causes of late-onset sepsis include enterococci (faecal streptococci), Listeria, Candida and miscellaneous rarer organisms.6-15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree