Study
Regimen
Diet
Success rate
Gremse et al. [15]
Oral sodium phosphate (45 mL/1.7 m2) 6 pm and 6 am for am procedure
Clear liquid 24 h
18/19
da Silva et al. [14]
Oral sodium phosphate (22.5 mL if <30 kg, 45 mL if >30 kg) pm and 5 am for am procedure
Clear liquid after first dose
10/14
Pinfield et al. [18]
Sodium picosulfate with magnesium citrate (2.5 g <2 year, 5 g 2–5 year, 10 g >5 year per dose) 24 and 18 h pre procedure
Clear liquid for 24 h
32/32 (3 vomited)
Dahshan et al. [17]
Magnesium citrate and X-prep
Clear liquid for 48 h
Table 20.2
Bowel preparation for children undergoing colonoscopy
Clear fluids for preceding 24 h (12 h for infants receiving no solid intake) | ||
|---|---|---|
5 pm | Senokot | 1–2 mg/kg (max 30 mg) |
Sodium picosulfate | 2.5 g if <1 year | |
5 g if 1–4 years | ||
10 g if >4 years | ||
6 am | Repeat sodium picosulfate dose | |
1 h before procedure | Phosphate enema (1/2 if <1 year) | |
The benefit of an intravenous antispasmodic agent administered directly before the ileocolonoscopy has recently been demonstrated [20]. Hyoscyamine 0.5 mg was given in this study of adults. An alternative could be hyoscine 20 mg administered intravenously. The use of such an agent given just prior to colonoscopy is determined by personal preference. Their use may facilitate ease of luminal visualization, but it also may increase the compliance of the colon, theoretically allowing a greater chance of loop formation. They are certainly of benefit in spastic colonic situations. It should be remembered that they work only for a short period of time, however, perhaps as short as 5 min and they may be re-administered in certain situations, such as when one needs to relax a haustral fold if a polyp is just beyond and obscured by it, or occasionally when one needs to relax a spastic ileocecal valve.
Monitoring and Sedation
“Sedation and analgesia” comprise a continuum of states ranging from minimal sedation (anxiolysis) through general anesthesia. Moderate sedation is a medically controlled state of depressed consciousness that allows protective reflexes to be maintained, retains the patient ability to maintain the airway independently and continuously. Deep sedation is a medically controlled state of depressed consciousness or unconsciousness from which the patient is not easily aroused and accompanied by a partial or complete loss of protective reflexes with inability to maintain a patent airway. General anesthesia is a controlled state of unconsciousness accompanied by a loss of protective reflexes [21, 22].
The aims of adequate sedation include patient safety, anxiolysis, analgesia, amnesia as well as adequate endoscopic examination, time and cost-efficiency [23]. Debate has surrounded the relative merits and safety of sedation and general anesthesia for upper gastrointestinal endoscopy and ileocolonoscopy in children for several years [24–26].
The risks of general anesthesia include those associated with intubation and administration of an anesthetic, which can be minimized by proper preparation and good intubation technique. General anesthesia can also be administered without intubation (intravenous propofol) or with the use of laryngeal mask. The benefits include complete amnesia and total avoidance of pain to the patient and as well as freeing the endoscopist from managing airway, monitoring vital signs and recovering the patient [23].
IV-S usually consists of a narcotic (meperidine or fentanyl) and a benzodiazepine (diazepam or midazolam), the former for analgesia and the latter for anxiolysis and amnesia. Ketamine and midazolam has been used with reportedly lesser side effects [27]. Table 20.3 lists some of the commonly used sedation regimens with the reversal agents. IV-S has been argued to be safe, effective and less costly in comparison to general anesthesia with successful sedation achieved in more than 95% of elective procedures [28, 29]. However careful monitoring of IV-S throughout the procedure is important [30, 31]. In spite of the advantages of IV-S pediatric gastrointestinal endoscopy has moved toward general anesthesia since it is now acknowledged that to get the requisite cooperation, and therefore a properly conducted procedure with minimum distress to the child, deep sedation is usually necessary. It is further recognized that there are attendant safety issues of airway maintenance in this situation, and at the very least, a specific individual with appropriate advanced pediatric life support skills should be responsible for the child’s cardio-respiratory welfare during such a procedure. The vast majority of pediatric gastrointestinal endoscopy under the age of 8 years in the United Kingdom, for instance, now occurs under general anesthesia.
Table 20.3
Sedation and reversal medications commonly employed in pediatric endoscopy
Midazolam | IV initial dose 0.05–0.1 mg/kg, then titrate to max 0.3 mg/kg or 10 mg whichever lower [21], 0.75 mg/kg or 15 mg whichever lower |
Fentanyl | IV initial dose 0.5–1.0 μg/kg, then titrate to max 5 μg/kg [48] |
Meperidine/pethidine | IV initial dose 0.5 mg/kg, then titrate to max 2 mg/kg or 75 mg whichever lower [120] |
Flumazenil | IV 0.02 mg/kg (max 0.2 mg) and repeat every minute to max of 0.05 mg/kg or max 1 mg [48] |
Naloxone | IV 0.1 mg/kg (max 2 mg) and repeat every 2–3 min to max 10 mg [48] |
When a child is sedated, resuscitation equipment should be easily accessible, and one or more people trained in pediatric advanced life support should be responsible for maintaining the airway and monitoring respiration, heart rate, blood pressure, and oxygen saturation [21, 32]. Sedation of younger children can be aided by environmental comforts such as a soothing voice or dimmed lights [33]. In all age groups, it is often necessary to use deep sedation because of the pain that can be associated with this procedure [34]. With deep sedation, it is clear that the risks are significant, including hypotension, respiratory compromise, and even respiratory arrest.
Recent studies examining the safety of general anesthesia for day-case ileocolonoscopy in children refute claims that there may be more risk of perforation because the operator cannot judge the degree of discomfort as a marker of impending traction injury [35, 36]. There is indeed a lack of evidence to support the contention that there is a higher complication rate with a general anesthetic than with sedation [37]. In fact, the airway is protected in a more effective and safer manner than with sedation, especially in upper endoscopy, with an improved operator satisfaction [38].
Preprocedural medication with a benzodiazepine has been found to be useful in reducing patient anxiety and improve patient and parent acceptance of the procedure without any adverse effects [39].
Endoscopic Techniques in Inflammatory Bowel Disease
Upper Gastrointestinal Endoscopy
While it is generally accepted that ileocolonoscopy and biopsy has a central role in the initial diagnosis and differentiation of IBD [40], it is now widely accepted that esophagogastroduodenoscopy (EGD) also has a definitive place in the initial diagnostic work up [41, 42]. Upper gastrointestinal involvement was long thought to be relatively uncommon [43, 44]; hence EGD was not routinely performed. Presence of upper gastrointestinal symptoms have been commonly considered as an indication to endoscopy [45]. Typically described upper gastrointestinal symptoms include dysphagia, pain when eating, nausea and/or vomiting, and aphthous lesions of the mouth. Diagnosis was often based on radiological changes [46] and EGD was reserved for those patients who had upper GI symptoms and or uncertain diagnosis. However, several studies have shown a higher incidence of microscopic mucosal disease in the upper GI tract [45, 47–51] than previously thought even in the absence of any upper GI symptoms [42, 52]. Cameron [47] in a prospective study described histological abnormalities on gastro-duodenal biopsies in 71% of patients with CD. Histological abnormalities including granulomas are seen even when the gross appearance of the tissue is normal [41, 45, 51–53]. Therefore, it is important to take biopsies from several sites in the upper gastrointestinal tract even if they appear normal endoscopically.
In a prospective 3-year study involving 420 IBD patients from 27 centers, EGD was performed in 237 patients. Eighty percent of patients with CD had macroscopic and/or histological changes in the upper GI tract while in 9% of the patients EGD was helpful in making a definitive diagnosis [54]. In another prospective single-center study, 24 of 45 patients with CD had an upper GI involvement and presented at an younger age, had more severe disease and were more likely to have extra-intestinal manifestations [55].
Esophageal disease in CD (Fig. 20.1) can vary from small erosions to transmural involvement resulting in perforation and fistulization into adjacent organs [56]. Granulomas are reported in 20–39% of esophageal biopsies in patients with CD [57, 58]. Other findings include erythema, ulceration, polypoid lesions, pseudomembranous formations, strictures, and mucosal bridges [57, 59–62]. Endoscopic findings in the stomach and duodenum include linear and serpigenous ulcers, diffuse superficial ulcers, aphthous ulcers, nodularity, cobblestone appearance, rigidity of the GI wall and narrowing of the lumen [63].
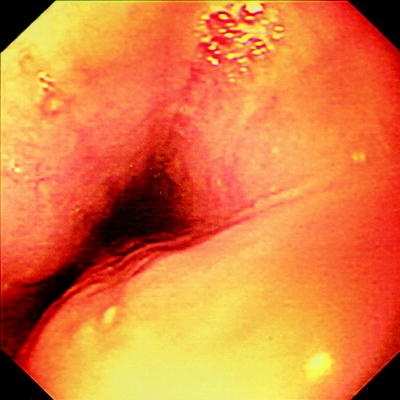

Fig. 20.1
Crohn disease of the esophagus showing discrete ulcers
Focal enhanced gastritis as an important feature of CD was first described by Schmitz-Moorman et al. [64] (Fig. 20.2). Further several studies confirmed this finding with a positive predictive value of 71–94% [48, 50, 65, 66]. Parente et al. [66] found focal antral gastritis more frequently in Helicobacter pylori negative adults with CD than in those with UC or in noninflammatory bowel conditions. Also they reported focal antral gastritis to be specific in 84% of patients with CD. While the presence of focal enhanced gastritis is suggestive of Crohn disease it is not pathognomonic of the condition.
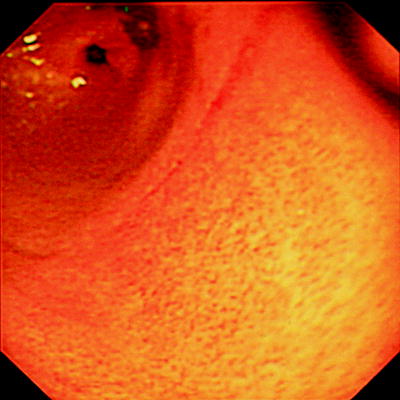

Fig. 20.2
Focal enhanced gastritis in Crohn disease
The presence of noncaseating granuloma is characteristic of CD. Granulomas are found in 7–68% of patients with CD in the upper GI tract [41, 45, 57, 63, 64] and often help in making a definitive diagnosis when none are found at other sites. Noncaseating granuloma in the upper gastrointestinal tract tends to occur in the superficial mucosa than in the muscularis or the serosal layers in comparison to ileal Crohn.
Ulcerative colitis conventionally was thought to involve only the colon and possibly ileum (backwash ileitis). However, it is increasingly recognized that features of inflammation in the upper GI tract [42, 50, 51, 67, 68] are seen in UC. Ruuska et al. [51] in a prospective study reported either macroscopic or histological upper gastrointestinal lesions in 75% of patients with UC. Abdullah et al. [41] also reported an incidence of 70% of histological abnormalities in the upper GI tract in patients with UC. Tobin et al. [50] in a controlled blinded study described esophagitis in 72% and 50% of patients with CD and UC, respectively. Gastritis was, however, more common and seen in 92% of CD and 69% of UC.
Ileocolonoscopy
Equipment
Most modern units employ adult and pediatric videocolonoscopes, and the general technical specifications for the pediatric instruments differ little between manufacturers (Table 20.4). When and in whom to use a pediatric colonoscope is mainly a matter of personal preference. We use personal judgment based on age and/or body weight. In general terms, the lower limit for the adult colonoscope is 3–4 years of age and/or 12–15 kg. The extra stiffness of the adult versions diminishes the likelihood of forming sigmoid loops, but extra care must then be taken, especially in younger children and with general anesthesia, not to advance against undue resistance, to avoid the unlikely complication of colonic perforation. The larger diameter of the adult colonoscopes can also lead to problems of maneuverability within the smaller colonic lumen of a young child. The variable stiffness colonoscope (see Table 20.4) may negotiate some of these problems. A control dial on the upper shaft of this small-diameter colonoscope (Olympus XCF-240AL/I) allows an increase in the stiffness of the insertion tube when passing through the sigmoid and transverse colon to avoid looping [69].
Table 20.4
Technical specifications of various pediatric colonoscopes
Parameter | Fujinon (EC-410 MP15) | Olympus (PCF 240L/I) | Olympus variable stiffness (CF 240AL/I) | Pentax (EC-3440PK) |
|---|---|---|---|---|
Angle of vision | 140° | 140° | 140° | 140° |
Depth of field | 6–100 mm | 4–100 mm | 3–100 mm | 6–100 mm |
Distal end | 11 mm | 11.3 mm | 12.2 mm | 11.5 mm |
Insertion tube | 11.1 mm | 11.3 mm | 12.0 mm | 11.4 mm |
Channel | 2.8 mm | 3.2 mm | 3.2 mm | 3.8 mm |
Angle up/down | 180°/180° | 180°/180° | 180°/180° | 180°/180° |
Angle right/left | 160°/160° | 160°/160° | 160°/160° | 160°/160° |
Working length | 1,520 mm | 1,330 mm (I) 1,680 mm (L) | 1,330 mm (I) 1,680 mm (L) | 1,500 mm |
More recently, magnifying colonoscopes have been developed, and their value in combination with dye spray or chromoscopy in various gastrointestinal diseases has been described [70]. For instance, the decrease in the number of cryptal openings in ulcerative colitis can be observed and correlated to disease activity [71], but this does not substitute for histological assessment.
Ileocolonoscopy Basic Technique
Getting Started and Patient Positioning
The patient is usually positioned in the left lateral knee to chest position, although some operators prefer the right lateral position, citing easier sigmoid negotiation. Certainly, if the procedure is not subsequently allowing easy access to the splenic flexure, then patient repositioning from one side to the supine and then to the other side may be advantageous. In general, frequent turning of the patient is conducive to easier ileocolonoscopy as a whole and is to be advocated. An assistant stands on the operator’s left to administer any abdominal pressure that may subsequently be deemed necessary to control, or try to prevent, loop formation in the sigmoid or transverse colon.
Practical Tips in Ileocolonoscopy
One important “trick” in learning ileocolonoscopy is to grasp the concept of the lumen and the positions of a clock face. For instance, if the lumen is at 9 o’clock, then to enter this requires anticlockwise rotation combined with upward deflection of the scope tip from the “neutral” position of 12 o’clock. Similarly, a combination of upward deflection of the tip with clockwise rotation of the colonoscope will allow entry of the lumen, suggested by a dark crescent, if seen at anywhere clockwise from 12 o’clock to 6 o’clock. Obviously, one may equally use downward tip deflection combined with the opposite rotatory control to that with upward tip deflection, and the execution and teaching of this concept are at personal discretion. With either approach, this is the most important maneuver that can be learned to assist in three-dimensional spatial orientation in the colon.
Prolonged “side viewing” of the bowel wall as it slides by should be avoided. Generally, the only place where, very temporarily, the lumen should be out of view is the occasional difficult negotiation of the splenic flexure. The patient’s position may be changed throughout the procedure to facilitate removal of loops and to allow a better view of the lumen because the gravity-dependent material in the colonic lumen changes position. Relatively minimal insufflation of air is desirable in the sigmoid colon because excess air may increase the chance of sigmoid loop formation (carbon dioxide provided by a specific commercially available delivery system attached to the colonoscope may be preferable because it is absorbed much more quickly decreasing pain and the very unlikely chance of perforation; see “Complications”).
In handling the colonoscope, it is good practice to have a flat unimpeded surface on which to place the remainder of the colonoscope that is not yet inserted; this is particularly important since any resistance encountered by the operator to forward advancement of the colonoscope can be attributed to colonic obstruction or loop formation within the child’s colon. Hence, relatively quickly, the trainee can acquire a realization of the normal expected resistance to scope advancement. This, in turn, allows understanding of the likelihood of loop formation, without any external resistance to scope advancement, causing confusion with regard to the behavior of the colonoscope within the patient.
Generally, in ileocolonoscopy, gentle scope advancement with clear lumen visualization is desirable, and, usually, only the forefinger and thumb will be required to advance the colonoscope. If greater pressure is required, then the operator is not performing an optimum procedure, and loop formation is likely to have occurred.
Rectal Intubation
Prior to any colonoscopy, it is considered good practice to perform an anal and then a rectal digital examination, the latter to avoid missing, by colonoscopy, very low-lying rectal polyps (although, where possible, retroflexion of the colonoscope in the rectum should occur prior to removal of the instrument to avoid missing lesions close to the anal margin). Adequate water-soluble lubrication, avoiding the tip of the instrument, allows easy passage into the rectum, which can occur with or without digital guidance from the operator’s index finger. Posterior positioning of the tip and air insufflation will allow visualization of the rectal mucosa and the three semilunar folds, or valves of Houston, occurring on alternating sides of the lumen. Subsequently, direct visualization of the bowel lumen is mandatory, except in some circumstances at the splenic flexure. If, at any point, a maneuver results in loss of visualization of the lumen, then reversal of what the operator has just done will often return the lumen to view; if not, the gentle scope retraction combined with minor tip deflections using the wheels and minor rotation of the scope in both directions will usually result in reorientation in the lumen. Obviously, if luminal contents are blocking the view, then lens cleaning will help.
Sigmoid and Descending Colon
Gentle torquing of the shaft clockwise and anticlockwise combined with upward or downward tip deflection and scope advancement is ideal for negotiating the sigmoid colon—the so-called “torque-steering” technique. The initial sigmoid fold or valve can usually be passed by 90–120 of anticlockwise torsion. The different loops encountered in the sigmoid are demonstrated in Fig. 20.3. A so-called N loop may be overcome by trans-abdominal pressure by an assistant on the apex of the loop pushing toward the feet (see Fig. 20.3a). This often allows a so-called α loop to form, which can usually be tolerated as the instrument advances toward the splenic flexure (see Fig. 20.3b). Reducing an α loop is accomplished by initial clockwise rotation and then slow removal of the colonoscope, keeping the lumen in the center of the field of vision. This may not be possible until the transverse (or even ascending) colon has been entered, in which case, hooking the tip of the scope over the splenic flexure may assist it. Paradoxical movement of the tip forward may be observed as the instrument is withdrawn and the bowel “concertinas” over the colonoscope. Abdominal pressure in the left iliac fossa may be helpful. The sigmoid and descending colons are relatively featureless, with less haustral folds than more proximally in the colon.
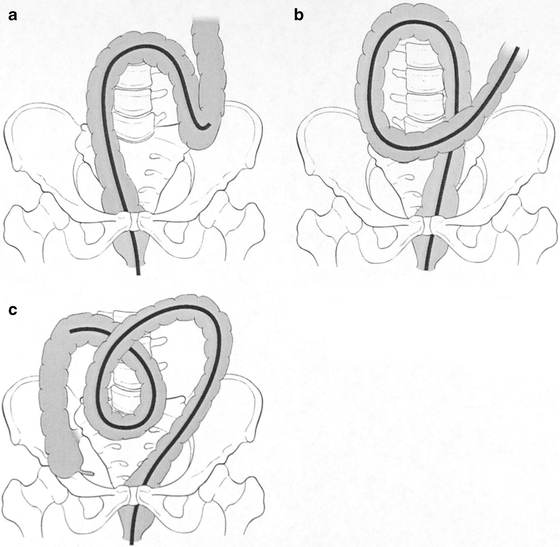

Fig. 20.3
Diagram of colonoscope sigmoid loops that may form. (a) An N loop in the sigmoid colon; (b) an α loop in the sigmoid colon; (c) a γ loop in a redundant transverse colon
Splenic Flexure and Transverse Colon
Nonlooped colonoscope length used at this point might be 40 cm in older children and even 20–25 cm in those under the age of 3–4 years. This is valuable in determining whether a loop is present. At the splenic flexure, the spleen may then be seen as a dark blue transmural discoloration. When negotiating the splenic flexure, the most successful combination of tip maneuvers is that of clockwise, right, and up followed by anticlockwise after passing the flexure. Occasionally, placing the patient in the right decubitus position may assist. The transverse colon is recognized by the triangular haustral folds and is usually easily passed (Fig. 20.4). Supine or right decubitus positioning may ease this. A loop in the shape of a “U” may occur in a dependent transverse colon, which is supported by abdominal pressure. The more difficult γ loop may occur in a redundant transverse colon (Fig. 20.3c). In addition, a good bit of advice is to apply gentle suction as the tip is advanced again in an attempt to concertina a potentially long dependent transverse colon over the colonoscope, thus maintaining a relatively short colonoscope and, hence, good control and maneuverability.
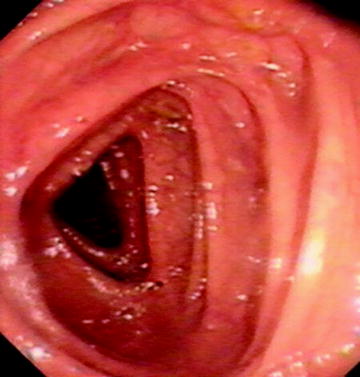

Fig. 20.4
Normal triangular appearance of transverse colon
Hepatic Flexure and Ascending Colon
Nonlooped colonoscope length used at this point might be 60 cm in older children and even 40 cm in those under the age of 3–4 years. This is valuable in determining whether a loop is present. The hepatic flexure is also recognized by the dark, usually blue, discoloration seen through the bowel wall, and positional change to the supine or right decubitus may again facilitate identification of the lumen. The combination of right, up, and clockwise followed by anticlockwise rotation and suction down into the ascending colon once around the sharply angled hepatic flexure is usually the most effective maneuver, but various combinations, including position change and scope withdrawal, may be required. Another tip is to remember that it is easy to be too far advanced into the vault of the hepatic flexure, leading to advance into a blind end, and often slight withdrawal of the instrument may reveal the fact that one is trying to negotiate this blind-ended area. The two or three sharp folds then observed may then be most successfully negotiated by tip deflection using both up/down and left/right wheels with minimal advancement of the scope. This is most easily performed in the supine patient position, however.
Once the hepatic flexure is negotiated, the transverse colonic γ loop may be reduced with anticlockwise or clockwise rotation followed by withdrawal of the colonoscope and suction. Loop withdrawal is essentially informed guesswork initially. Studies with the colon Map guider, based on using a colonoscope with an inbuilt electromagnetic loop that allows accurate real-time colonoscope three-dimensional positioning by detection using an external positioning device and displayed on a screen next to the patient, have shown that even expert colonoscopists get the type of loop present wrong in half of the cases [74–76]. Once one starts to remove the loop, using rotation only initially, a tip is to gently start to remove the colonoscope and try to determine whether within-patient resistance is increasing or whether the colonoscope is trying to push your hand away from the patient as the loop unfolds. Usually, trying clockwise or anticlockwise combined with instrument withdrawal will, with experience, allow early determination of which rotation direction is likely to be successful in “delooping” the colonoscope. It is best to try to maintain good luminal vision during this procedure, but, not infrequently, the lumen is lost; however, if this loop removal technique is effective, it is then not unusual to find oneself then looking at the appendiceal orifice and hence the cecum because the scope will have naturally traveled down the ascending colon. It is important to remember that the ascending colon, which in children is of variable length, may be as short as 5 cm in some younger patients.
Cecum
Three useful ways to ensure that one has reached the cecum are as follows:
1.
Observing the colonoscopic illumination in the right iliac fossa (using the specific high-intensity light transillumination application available with some colonoscopes is not usually necessary in children, excepting with some obese adolescents, for whom it can be helpful when applied in a dark environment).
2.
Digitally indenting the abdominal wall over the right iliac fossa and observing the corresponding effect on the colonic wall with the colonoscope.
3.
Identifying the triradiate fold, appendiceal orifice, and (especially if gas bubbles or ileal effluent are being excreted from it) the typical two lips-like appearance of the ileocecal valve.
A good maxim is that if there is any doubt in the operator’s mind about having reached the cecum, then one is usually at the hepatic or even splenic flexure. Only about 80 cm of scope from the anus is needed when all loops are removed in an adult, and in smaller children, only 40–60 cm may be needed. This assumes normal anatomy of the ascending colon and cecum. Obviously, cecal strictures can confuse the picture.
Ileal Intubation and its Importance
The ileocecal valve is present approximately 1–4 cm distal to the appendiceal orifice opening into a smooth asymmetric fold and opens perpendicular to the axis of the colon. Figure 20.5 shows the steps of the easiest technique for ileal intubation. Removal of any colonic loops is important to allow for a responsive scope with no paradoxical movement. Figures 20.5b and 20.6 show the valve maneuvered to the 6 o’clock position, usually after clockwise rotation of the scope and wheel-tip deflection to maintain a centered cecal view. Anticlockwise rotation can also be used but is less efficient. If too much gas is present, then the cecum may be “tented,” and this should be suctioned prior to an ileal intubation attempt. Figures 20.5c and 20.7 show the insertion of the biopsy forceps such that just the tip or the first few millimeters are visibly exposed beyond the end of the scope. The scope is then inserted just beyond the fold (using the downward deflecting wheel with the scope as above already in the 6 o’clock position), and the tip is inclined downward so that the forceps gently press into the wall. Slight left inclination may be required at this point to open the valve like a pair of lips on slight withdrawal of the scope (Fig. 20.5c). Once the valve is opened, the scope may be passed into the ileum with further downward deflection. Often this is facilitated by small right and left deflections with an assistant pressing on the abdomen over the transverse colon to support a dependent transverse and also prevent loop formation. In the absence of ileocecal valve strictures, and with practice, this technique will allow an ileal intubation rate of 100%. Perforation of the cecum or ileum with this technique is a theoretical concern raised by some observers unfamiliar with this technique, but this has not occurred in our experience of over 5,000 ileocolonoscopies and is extremely unlikely.
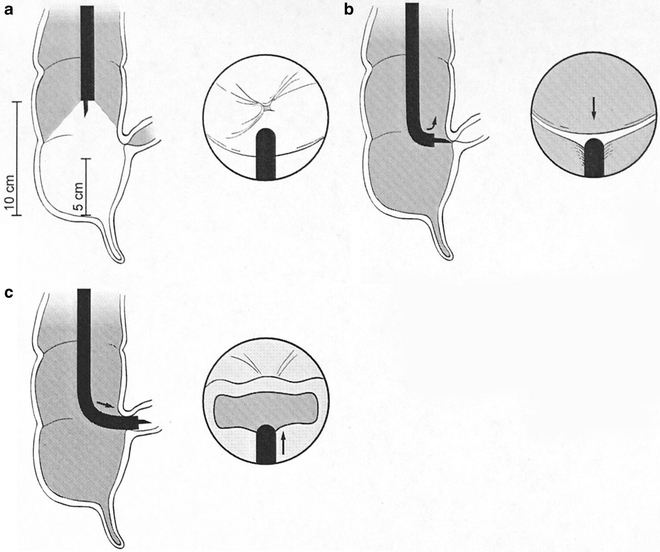
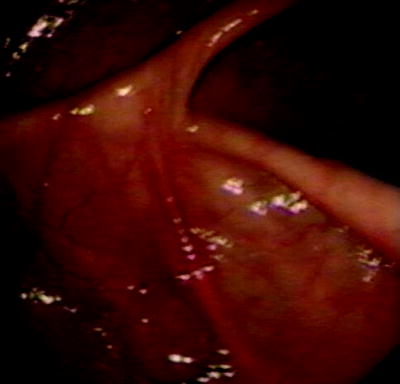
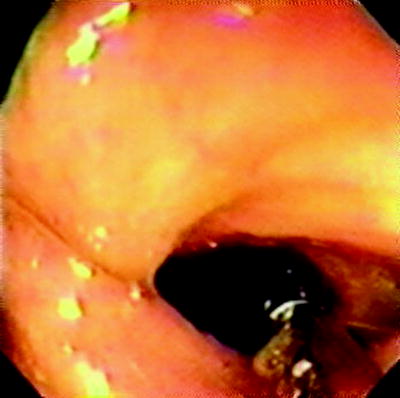

Fig. 20.5
(a) Identification of cecum with triradiate fold, appendicele orifice and ileocecal valve; (b) ileocecal valve at 6 o’clock position; (c) forceps opening up ileocecal valve with downward defection of colonoscope tip

Fig. 20.6
Ileocecal valve at 6 o’clock

Fig. 20.7
Ileocecal entry using forceps
An alternative technique is “blind” intubation of the ileocecal valve. This involves the same positioning of the valve at 6 or 9 o’clock and then slowly withdrawing the scope back from just beyond the valve’s fold while insufflating with air and deflecting the scope tip downward. The disadvantage of this technique is that it is not under direct vision.
Ileum
The ileal mucosa will have the typical velvet-like appearance of small bowel (Fig. 20.8), with the presence of smoother raised areas, which are Peyer patches, and, occasionally, lymphonodular hyperplasia of varying degrees (Fig. 20.9). Villi are more easily seen if the lumen is flooded with water. The ileal surface is shown in greater relief with a spray of standard blue or black ink (methylene blue in a 1:20 dilution may also be used); this is also useful in showing the detail of sessile polyps in the colon. Deeper intubation of the ileum by either technique is similar to duodenal negotiation during upper gastrointestinal endoscopy, and up to 40 cm of ileum can be observed.
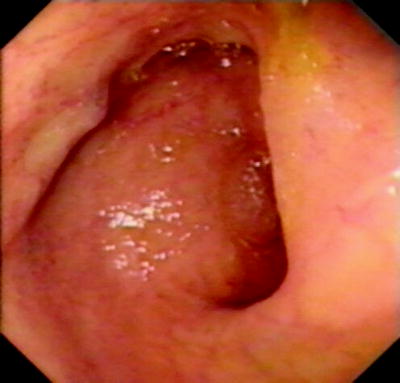
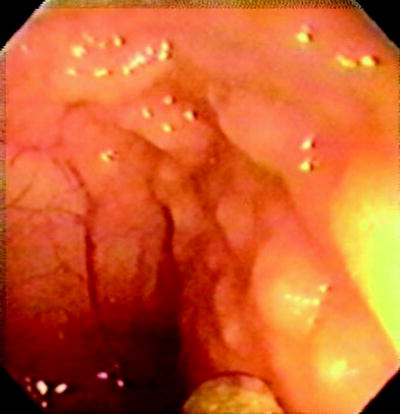

Fig. 20.8
Normal appearance of terminal ileum

Fig. 20.9
Lymphonodular hyperplasia of the terminal ileum
It is pertinent here to discuss the diagnostic need for entering the ileum in children suspected of IBD. Williams et al., in 1982 [77], reported their experience of total ileocolonoscopy in children in which the terminal ileum was examined in 63 patients. In six children, ileitis detected by ileocolonoscopy was the sole finding of Crohn disease, which was previously unrecognized by radiological contrast studies. Lipson et al. compared ileoscopy and barium studies, with an endoscopy specificity of 0.96 for diagnosis of Crohn disease in the terminal ileum [78]. In 14 of 46 children, ileoscopy revealed diagnosis, which would otherwise have been missed. This study also made clear that the endoscopic appearances could be completely normal, yet the diagnosis of Crohn disease could be made histologically by the presence of granulomata. Also, a pronounced lymphoid hyperplasia pattern was present radiologically in 24% of children and would have been a source of error in two cases had contrast radiographs been relied on to make the diagnosis without ileoscopy. More recently, Deere et al. showed that sigmoid, colonic, and rectal biopsies confirm the diagnosis of IBD in only 60% of cases, and diagnosis based on morphological criteria was possible in only 85% of cases when the cecum was reached without ileal intubation [79]. Geboes et al. assessed 300 patients, including adolescents and children, and found endoscopic and histological ileal lesions in 123 and 125, respectively, of whom no colonic disease was present in 44 [80]. Ileal biopsies were essential for the diagnosis in 15 patients and contributory in 53. There are, of course, other reasons apart from the principal one, that is, diagnosis of chronic IBD, for entering the ileum in children. For instance, ileoscopy will facilitate diagnoses of other causes of ileitis such as infection with tuberculosis or Yersinia [81]. In addition, therapeutic dilation of short terminal ileal strictures by per endoscopic balloon catheter may be attempted.
Endoscope Withdrawal
A more careful inspection of the colon is necessary on withdrawal of the scope, especially for the presence of polyps, which may have remained hidden behind a haustral fold during the initial insertion of the scope. Biopsies should be taken from all areas, including normal-looking mucosa to allow for accurate histological diagnosis. Biopsy technique is similar to EGD, with the exception that many colonoscopic biopsy forceps have a central barb, allowing more than one biopsy to be taken each time the forceps are passed.
Lastly, before removing the scope from the anus, a retroflexion maneuver obtained by maximum upward and right or left tip deflection and slight advancement of the scope into the rectal vault, followed by rotation clockwise and anticlockwise through 180° completes the examination. This is necessary to observe the ano-rectal junction and distal rectum. Distal ulcers, inflammation, or even polyps can be missed if this is not done.
Dilation of Strictures
Through-the-scope balloon dilators are appropriate for ileocolonic dilation, employing the same concept and method as for upper gastrointestinal strictures, employing radiological screening control. Long-term symptomatic relief can be afforded in some carefully selected patients, including adolescents in reported studies [82, 83]. Pressures of 35–50 psi in balloons of 12–18 mm are available. Theoretically, as for neoplastic or diverticulitis-associated strictures in adults, stent placement could be used as a last resort in IBD-type strictures, but there are no reported cases of this occurring in childhood as yet.
Complications of Ileocolonoscopy
Complications, excluding those due to sedation, are summarized in Table 20.5. Complications are more common following therapeutic procedures. The literature to date reveals over 3,000 colonoscopies under 20 years of age reported, with five perforations—four postpolypectomy and one in a patient with severe ulcerative colitis. Ten procedure-related minor complications are noted, including four small postpolypectomy hemorrhages, three cases of postprocedure abdominal pain with spontaneous resolution, one common peroneal nerve palsy secondary to periprocedure positioning, and two with a postprocedure fever for more than 24 h [35, 36, 40, 84–88]. This equates to a complication rate owing to the procedure itself of approximately 0.3% and, without polypectomy, of about 0.05%. This is in keeping with the British definition of “minimal” risk and the American definition of “minor risk over minimal” [89].
Table 20.5




Procedure-related and postprocedure complications in pediatric colonoscopy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


