Endometriosis is defined as the presence of endometrial tissue (glands and stroma) outside the uterus. The most frequent sites of implantation are the pelvic viscera and the peritoneum. Endometriosis varies in appearance from a few minimal lesions on otherwise intact pelvic organs, to massive ovarian endometriotic cysts that distort tubo-ovarian anatomy and extensive adhesions involving bowel, bladder, and ureter. It is estimated to occur in 10% of reproductive-age women and is associated with pelvic pain and infertility. Considerable progress in understanding the pathogenesis, spontaneous evolution, diagnosis, and treatment of endometriosis has occurred. The European Society for Human Reproduction and Embryology (ESHRE) guidelines for the clinical management of endometriosis are published and regularly updated to present emerging clinical evidence (1).
Epidemiology
Prevalence
Endometriosis is found predominantly in women of reproductive age but is reported in adolescents and in postmenopausal women receiving hormonal replacement (2). It is found in women of all ethnic and social groups. Estimates of the frequency of endometriosis vary widely, but the prevalence of the condition is assumed to be around 10% (3,4). Although no consistent information is available on the incidence of the disease, temporal trends suggest an increase among women of reproductive age (4).
In women with pelvic pain or infertility, a high prevalence of endometriosis (from a low of 20% to a high of 90%) is reported (5,6). In women with unexplained subfertility with or without pain (regular cycle, partner with normal sperm), the prevalence of endometriosis is reported to be as high as 50% (7). In asymptomatic women undergoing tubal ligation (women of proven fertility), the prevalence of endometriosis ranges from 3% to 43% (8–13). This variation in the reported prevalence may be explained by several factors. First, it may vary with the diagnostic method used: laparoscopy, the operation of choice for diagnosis, is a better method than laparotomy for diagnosing minimal to mild endometriosis. Second, minimal or mild endometriosis may be more thoroughly evaluated in a symptomatic patient given general anesthesia than in an asymptomatic patient during tubal sterilization. Third, the interest and experience of the surgeon is important because there is a wide variation in the appearance of subtle endometriosis implants, cysts, and adhesions. Most studies that evaluate the prevalence of endometriosis in women of reproductive age lack histologic confirmation (8–10,14–19).
Risk and Protective Factors
The following are identified risk factors for endometriosis: infertility, red hair, early age at menarche, shorter menstrual cycle length, hypermenorrhea, nulliparity, müllerian anomalies, birth weight (less than 7 pounds), one of multiple fetal gestation, diethylstilbestrol (DES) exposure, endometriosis in first-degree relative, tall height, dioxin or polychlorinated biphenyls (PCB) exposure, a diet high in fat and red meat, and prior surgeries or medical therapy for endometriosis (20). Prior use of contraception or intrauterine device (IUD), or smoking is not associated with increased risk of endometriosis (21,22). Protective factors against the development of endometriosis include multiparity, lactation, tobacco exposure in utero, increased body mass index, increased waist-to-hip ratios and exercise, and diet high in vegetables and fruits (20). Some evidence suggests that women with a “pinpoint cervix” have an increased risk for endometriosis, but more studies are needed to confirm this observation (23).
Endometriosis and Cancer
Several publications link endometriosis with an increased risk for certain gynecologic and nongynecologic cancers (24,25). These associations are controversial and no data exist to inform clinicians regarding the best management of patients who might be at risk of developing such cancers (1). Endometriosis should not be considered a medical condition associated with a clinically relevant risk of any specific cancer (26). Data from large cohort and case-control studies indicate an increased risk of ovarian cancers in women with endometriosis. The observed effect sizes are modest, varying between 1.3 and 1.9 (27). Evidence from clinical series consistently demonstrates that the association is confined to the endometrioid and clear-cell histologic types of ovarian cancer (28). A causal relationship between endometriosis and these specific histotypes of ovarian cancer should be recognized, but the low magnitude of the risk observed is consistent with the view that ectopic endometrium undergoes malignant transformation with a frequency similar to its eutopic counterpart (29). Evidence for an association with melanoma and non-Hodgkin’s lymphoma is increasing but needs to be verified, whereas an increased risk for other gynecologic cancer types is not supported (28).
Etiology
Although signs and symptoms of endometriosis were described since the 1800s, its widespread occurrence was acknowledged only during the 20th century. Endometriosis is an estrogen-dependent disease. Three theories were proposed to explain the histogenesis of endometriosis:
No single theory can account for the location of endometriosis in all cases.
Transplantation Theory
The transplantation theory, originally proposed by Sampson in the mid-1920s, is based on the assumption that endometriosis is caused by the seeding or implantation of endometrial cells by transtubal regurgitation during menstruation (30). Substantial clinical and experimental data support this hypothesis (5,31). Retrograde menstruation occurs in 70% to 90% of women, and it may be more common in women with endometriosis than in those without the disease (8,32). The presence of endometrial cells in the peritoneal fluid, indicating retrograde menstruation, is reported in 59% to 79% of women during menses or in the early follicular phase, and these cells can be cultured in vitro (33,34). The presence of endometrial cells in the dialysate of women undergoing peritoneal dialysis during menses supports the theory of retrograde menstruation (35). Endometriosis is most often found in dependent portions of the pelvis—the ovaries, the anterior and posterior cul-de-sac, the uterosacral ligaments, the posterior uterus, and the posterior broad ligaments (36). The menstrual reflux theory combined with the clockwise peritoneal fluid current explains why endometriosis is predominantly located on the left side of the pelvis (refluxed endometrial cells implant more easily in the rectosigmoidal area) and why diaphragmatic endometriosis is found more frequently on the right side (refluxed endometrial cells implant there by the falciform ligament) (37,38).
Endometrium obtained during menses can grow when injected beneath abdominal skin or into the pelvic cavity of animals (39,40). Endometriosis was found in 50% of Rhesus monkeys after surgical transposition of the cervix to allow intra-abdominal menstruation (41). Increased retrograde menstruation by obstruction of the outflow of menstrual fluid from the uterus is associated with a higher incidence of endometriosis in women and in baboons (42–44). Women with shorter intervals between menstruation and longer duration of menses are more likely to have retrograde menstruation and are at higher risk for endometriosis (45). Menstruation is associated with intraperitoneal inflammation in both women and baboons, but a limited quantity of endometrial cells can be identified in peritoneal fluid during menstruation in women, possibly because endometrial–peritoneal attachment is reported to occur within 24 hours (46–48). Ovarian endometriosis may be caused by either retrograde menstruation or by lymphatic flow from the uterus to the ovary; metaplasia and bleeding from a corpus luteum may be a critical event in the development of some endometriomas (49–51).
Deeply infiltrative endometriosis, with a depth of at least 5 mm beneath the peritoneum, can present as nodules in the cul-de-sac, rectosigmoid, and bladder area and occurs with other forms of peritoneal or ovarian endometriosis (52). According to anatomic, surgical, and pathologic findings, deep endometriotic lesions originate intraperitoneally rather than extraperitoneally. The lateral asymmetry in the occurrence of ureteral endometriosis is compatible with the menstrual reflux theory and with the anatomic differences of the left and right hemipelvis (37). Adolescents and young women can have peritoneal disease (53). This observation, together with evidence from the development and spontaneous evolution of endometriosis in baboons, supports the notion that endometriosis starts as peritoneal disease and that the three different phenotypes and locations of endometriosis (peritoneal, ovarian, and deep) represent a homogenous disease continuum with a single origin (i.e., regurgitated endometrium), rather than three different disease entities, as advocated by some investigators (37,54,55).
Extrapelvic endometriosis, although rare (1% to 2%), may result from vascular or lymphatic dissemination of endometrial cells to many gynecologic (vulva, vagina, cervix) and nongynecologic sites. The latter include bowel (appendix, rectum, sigmoid colon, small intestine, hernia sacs), lungs and pleural cavity, skin (episiotomy or other surgical scars, inguinal region, extremities, umbilicus), lymph glands, nerves, and brain (56).
Coelomic Metaplasia
The transformation (metaplasia) of coelomic epithelium into endometrial tissue is a proposed mechanism for the origin of endometriosis. One study evaluating structural and cell surface antigen expression in the rete ovarii and epoophoron reported little commonality between endometriosis and ovarian surface epithelium, suggesting that serosal metaplasia is unlikely in the ovary (57). The results of another study involving the genetic induction of endometriosis in mice suggest that ovarian endometriotic lesions may arise directly from the ovarian surface epithelium through a metaplastic differentiation process induced by activation of an oncogenic K-ras allele (50).
Induction Theory
The induction theory is an extension of the coelomic metaplasia theory. It proposes that an endogenous (undefined) biochemical factor can induce undifferentiated peritoneal cells to develop into endometrial tissue. This theory is supported by experiments in rabbits but is not substantiated in women or nonhuman primates (58,59).
Genetic Factors
Increasing evidence suggests that endometriosis is partially a genetic disease. Recent findings that support this association include evidence of familial clustering in humans and in Rhesus monkeys, a founder effect detected in the Icelandic population, concordance in monozygotic twins, a similar age at onset of symptoms in affected nontwin sisters, a six- to nine-times increased prevalence of endometriosis among first-degree relatives of women compared with the general population, and a 15% prevalence of magnetic resonance imaging (MRI) findings suggestive of endometriosis in the first-degree relatives of women with stage III or IV disease based on the classification of the American Society of Reproductive Medicine (60). The induction of humanlike endometriosis by genetic activation of an oncogenic K-ras allele lends further support to the genetic basis of this disorder (50).
Population Studies
The risk of endometriosis is seven times greater if a first-degree relative is affected by endometriosis (61). Because no specific Mendelian inheritance pattern is identified, multifactorial inheritance is postulated. A relative risk for endometriosis of 7.2 was found in mothers and sisters, and a 75% (six in eight) incidence was noted in homozygotic twins of patients with endometriosis (62). In another study of twins, 51% of the variance of the latent liability to endometriosis may be attributable to additive genetic influences (63). Other investigators reported that 14 monozygotic twin pairs were concordant for endometriosis, and two pairs were discordant (64). Of these twin pairs, nine had moderate to severe endometriosis. A relationship was shown between endometriosis and systematic lupus erythematosus, dysplastic nevi, and a history of melanoma in women of reproductive age (65,66). Endometriosis is linked to the presence of individual human leukocyte antigens (67–69). Genome-wide association studies show that the risk of endometriosis is associated with a mutation on the short arm of chromosome 7 (7p15.2) in women of European ancestry and that this association is the strongest for moderate to severe disease (70).
Genetic Polymorphisms and Endometriosis
A number of studies investigated genetic polymorphisms as a possible factor contributing to the development of endometriosis. About 50% of the studies in one review demonstrated positive correlations between different polymorphisms and endometriosis (71). This relation was seen most clearly in groups 1 (cytokines and inflammation), 2 (steroid-synthesizing enzymes and detoxifying enzymes and receptors), 4 (estradiol metabolism), 5 (other enzymes and metabolic systems), and 7 (adhesion molecules and matrix enzymes). Group 8 (apoptosis, cell-cycle regulation, and oncogenes) seemed to be negatively correlated with the disease, whereas groups 3 (hormone receptors), 6 (growth factor systems), and especially 9 (human leukocyte antigen system components) showed a relatively strong correlation. As many results were contradictory, the review concluded that genetic polymorphisms might have a limited value in assessing possible development of endometriosis (71). Future studies should include large numbers of women with laparoscopically and histologically confirmed endometriosis and women with a laparoscopically confirmed normal pelvis as controls, taking into account ethnic variability.
Aneuploidy
Epithelial cells of endometriotic cysts are monoclonal on the basis of phosphoglycerate kinase gene methylation, and normal endometrial glands are monoclonal (72,73). In a comparison of endometriotic tissue with eutopic endometrium, flow cytometric DNA analysis failed to show aneuploidy (74). Studies using comparative genomic hybridization, or multicolor in situ hybridization, showed aneuploidy for chromosomes 11, 16, and 17, increased heterogeneity of chromosome 17 aneuploidy, and losses of 1p and 22q (50%), 5p (33%), 6q (27%), 70 (22%), 9q (22%), and 16 (22%) of 18 selected endometriotic tissues (75–77). In another study, trisomies 1 and 7, and monosomies 9 and 17 were found in endometriosis, ovarian endometrioid adenocarcinoma, and normal endometrium (78). The proportions of aneusomic cells were significantly higher in ovarian endometriosis compared with extragonadal endometriosis and normal endometrium (p < 0.001), suggesting a role of the ovarian stromal milieu in the induction of genetic changes, which may lead to invasive cancer in isolated cases (78).
Microsatellite DNA assays reveal an allelic imbalance (loss of heterozygosity) in p16 (Ink4), GALT, p53, and APOA2 loci in patients with endometriosis and in stage II of endometriosis (79). Another report found a loss of heterozygosity in 28% of endometriotic lesions at one or more sites: chromosomes 9p (18%), 11q (18%), and 22q (15%) (73).
Immunologic Factors and Inflammation
Although retrograde menstruation appears to be a common event in women, not all women who have retrograde menstruation develop endometriosis. The immune system may be altered in women with endometriosis, and it is hypothesized that the disease may develop as a result of reduced immunologic clearance of viable endometrial cells from the pelvic cavity (80,81). Endometriosis can be caused by decreased clearance of peritoneal fluid endometrial cells resulting from reduced natural killer (NK) cell activity or decreased macrophage activity (82). Decreased cell-mediated cytotoxicity toward autologous endometrial cells is associated with endometriosis (82–86). These studies used techniques that have considerable variability in target cells and methods (87,88). Whether NK cell activity is lower in patients with endometriosis than in those without endometriosis is controversial. Some reports demonstrate reduced NK activity and others found no increase in NK activity in women with moderate to severe disease (84–86,89–94). There is great variability in NK cell activity among normal individuals that may be related to variables such as smoking, drug use, and exercise (87).
In contrast, endometriosis can be considered a condition of immunologic tolerance, as opposed to ectopic endometrium, which essentially is self-tissue (80). It can be questioned why viable endometrial cells in the peritoneal fluid would be a target for NK cells or macrophages. Autotransplantation of blood vessels, muscles, skin grafts, and other tissues is extremely successful (83–85). There is no in vitro evidence that peritoneal fluid macrophages actually attack and perform phagocytosis of viable peritoneal fluid endometrial cells. High-dose immunosuppression can increase slightly the progression of spontaneous endometriosis in baboons (95). There is no clinical evidence that the prevalence of endometriosis is increased in immunosuppressed patients. The fact that women with kidney transplants, who undergo chronic immunosuppression, are not known to have increased infertility problems can be considered indirect evidence that these patients do not develop extensive endometriosis.
Substantial evidence suggests that endometriosis is associated with a state of subclinical peritoneal inflammation, marked by an increased peritoneal fluid volume, increased peritoneal fluid white blood cell concentration (especially macrophages with increased activation status), and increased inflammatory cytokines, growth factors, and angiogenesis-promoting substances. It is reported in baboons that subclinical peritoneal inflammation occurs during menstruation and after intrapelvic injection of endometrium (93). A higher basal activation status of peritoneal macrophages in women with endometriosis may impair fertility by reducing sperm motility, increasing sperm phagocytosis, or interfering with fertilization, possibly by increased secretion of cytokines such as tumor necrosis factor-α (TNF-α) (96–100). Tumor necrosis factor may facilitate the pelvic implantation of ectopic endometrium (99,100). The adherence of human endometrial stromal cells to mesothelial cells in vitro is increased by the pretreatment of mesothelial cells with physiologic doses of TNF-α (101). Macrophages or other cells may promote the growth of endometrial cells by secretion of growth and angiogenetic factors such as epidermal growth factor (EGF), macrophage-derived growth factor (MDGF), fibronectin, and adhesion molecules such as integrins (101–107). After attachment of endometrial cells to the peritoneum, subsequent invasion and growth appear to be regulated by matrix metalloproteinases (MMP) and their tissue inhibitors (108,109).
There is increasing evidence that local inflammation and secretion of prostaglandins (PG) is related to differences in endometrial aromatase activity between women with and without endometriosis. Expression of aromatase cytochrome P450 protein and mRNA is present in human endometriotic implants but not in normal endometrium, suggesting that ectopic endometrium produces estrogens, which may be involved in the tissue growth interacting with the estrogen receptor (110). Inactivation of 17β-estradiol is impaired in endometriotic tissues because of deficient expression of 17β-hydroxysteroid dehydrogenase type 2, which is normally expressed in eutopic endometrium in response to progesterone (111). The inappropriate aromatase expression in endometriosis lesions can be stimulated by prostaglandin E2 (PGE2). This reaction leads to local production of E2, which stimulates PGE2 production, resulting in a positive-feedback system between local inflammation and estrogen-driven local growth of ectopic endometrium (112).
The subclinical pelvic inflammatory status associated with endometriosis is reflected in the systemic circulation. Increased concentrations of C-reactive protein, serum amyloid A (SAA), TNF-α, membrane cofactor protein-1, interleukin-6 (IL-6), IL-8, and chemokine (C-C motif) receptor 1 (CCR1) are observed in peripheral blood samples of patients with endometriosis when compared with controls (113). This observation offers a basis for the development of noninvasive diagnostic tests.
Both hypothesis-driven research and system biology approaches using mRNA microarray and proteomic techniques studies show that eutopic endometrium is biologically different in women with endometriosis when compared to controls with respect to proliferation, apoptosis, angiogenesis, and inflammatory pathways (114–117). Several studies show a higher prevalence of nerve fibers and neurotrophic factors in the eutopic endometrium from women with endometriosis when compared to controls (46,118).
Environmental Factors and Dioxin
There is an increasing awareness of potential links between reproductive health, infertility, and environmental pollution. Attention was directed toward the potential role of dioxins in the pathogenesis of endometriosis, but the issue remains controversial. A meta-analysis concluded that there is insufficient evidence in women or in nonhuman primates that endometriosis is caused by dioxin exposure (119).
Human Data
A 1976 explosion of a factory in Seveso, Italy, resulted in the highest recorded levels of dioxin exposure in humans, but data are not published (120). The Seveso Women’s Health Study will correlate prospective individual data on exposure to dioxin with reproductive endpoints such as the incidence of endometriosis, infertility, and decreased sperm quality. One case-control study failed to show an association in the general population between endometriosis and exposure to PCB and chlorinated pesticides during adulthood. No differences in mean plasma concentrations of 14-PCB and 11-chlorinated pesticides were found between women with and those without endometriosis (121). In another study, increased exposure to dioxin-like compounds is associated with (moderate to severe) endometriosis in a case-control study in women (122). Genetic mechanisms may play a role in dioxin exposure and the development of endometriosis. Transcripts of the CYP1A1 gene, a dioxin-induced gene, are significantly higher (nine times higher) in endometriotic tissues than in eutopic endometrium (112). Other investigators report a similar expression of arylhydrocarbon receptor and dioxin-related genes (using semiquantitative reverse transcriptase polymerase chain reaction) in the endometrium from women with or without endometriosis (123). In Japanese women, no association was found between endometriosis prevalence or severity and polymorphisms for arylhydrocarbon receptor repressor, arylhydrocarbon (x2) receptor, and arylhydrocarbon nuclear translocator or CYP1A1 genes (124). Based on these data, there is insufficient evidence supporting the association between endometriosis and dioxin exposure in humans.
Primates
An initial retrospective case-control study reported that the prevalence of endometriosis was not statistically different (p = 0.08) between monkeys chronically exposed to dioxin during 4 years (11 of 14, 79%) and unexposed animals (2 of 6, 33%) after a period of 10 years. A positive correlation was found between the severity of endometriosis and dioxin dose, serum levels of dioxin, and dioxin-like chemicals (125,126). Two prospective studies evaluated the association between dioxin exposure and development of endometriosis in Rhesus monkeys. In one study, monkeys exposed over 12 months to low-dose dioxin (0.71 ng/kg/day) had endometriosis implants with smaller maximal and minimal diameters and similar survival rate when compared with endometriotic lesions in unexposed controls, suggesting no effect of dioxin on endometriosis (127). After 12 months of exposure to high-dose dioxin (17.86 ng/kg/day), larger diameters and a higher survival rate of endometriosis implants were observed in exposed Rhesus monkeys compared with unexposed controls. The second randomized study performed in 80 Rhesus monkeys compared those with no treatment with those treated with 0, 5, 20, 40, and 80 μg of Aroclor (1,254 kg per day) for 6 years. Endometriosis occurred in 37% of controls and in 25% of treated monkeys as determined by laparoscopy and necropsy data (128). No association was observed between endometriosis severity and PCB exposure. These data question the importance of dioxin exposure, except at high doses, in the development of endometriosis in primates.
Rodents
Continuous exposure to 2,3,7,8-tetrachlorodibenzo-P-dioxin inhibited the growth of surgically induced endometriosis in ovariectomized mice treated with high-dose estradiol. No correlation was observed between the dose of dioxin and survival of endometrial implants, adhesions, and serum E2 levels (129). In ovariectomized mice induced with endometriosis, similar stimulating effects of estrone and 4-chlorodiphenyl ether (4-CDE) were observed on survival rates of endometriotic mice, suggesting an estrogen-like effect of 4-CDE (130). Potential mechanisms mediating dioxin action to promote endometriosis in rodents are complex and probably different in rats and mice, and furthermore in women. The mouse appears to be a better model to elucidate these mechanisms, but both models have important limitations (131,132).
Stem Cells
Endometrial stem cells are identified, are bone marrow derived, can differentiate into neurogenic or pancreatic-β cells, may contribute to the development of endometriosis in a murine model, and their potential role in the pathogenesis of endometriosis needs to be investigated (133–136).
Future Research
The study of endometriosis is compounded by the need to determine the presence or absence of pathology. The pathogenesis of endometriosis, the pathophysiology of related infertility, and the spontaneous evolution of endometriosis are being studied. At the time of diagnosis, most patients with endometriosis had the disease for an unknown period, making it difficult to initiate clinical experiments that would determine the etiology or progression of the disease (31). Because endometriosis occurs naturally only in women and nonhuman primates, and invasive experiments cannot be performed easily, it is difficult to undertake properly controlled studies. There is a need for the development of a good animal model with spontaneous endometriosis. The main advantage of the rat and rabbit models used to study endometriosis is their low cost relative to primates, but the disadvantages are numerous (137–140). In both models, the type of lesion appears to be quite different from the variety of pigmented and nonpigmented lesions observed in women (137–139). Primates are phylogenetically close to humans, have a comparable menstrual cycle, are afflicted with spontaneous endometriosis, and when induced with endometriosis, develop macroscopic lesions that are similar to those found in human disease (41,141–145). Spontaneous endometriosis in the baboon is minimal and disseminated, similar to the different stages of endometriosis in women (141,146–148).
Immunomodulatory drugs inhibiting pelvic inflammation associated with endometriosis may offer new approaches to medical treatment and can be studied in these models (149–153). In a consensus workshop following the 10th World Congress on Endometriosis in 2008, it was agreed that multidisciplinary expertise is required to advance our understanding of this disease, and 25 recommendations for research were developed (154).
Diagnosis
Clinical Presentation
Endometriosis should be suspected in women with subfertility, dysmenorrhea, dyspareunia, or chronic pelvic pain, although these symptoms can be associated with other diseases. Endometriosis may be asymptomatic, even in women with more advanced disease, (i.e., ovarian endometriosis or deeply invasive rectovaginal endometriosis).
Risk factors for endometriosis include short cycle length, heavier menstruation, and longer flow duration, probably related to a higher incidence of retrograde menstruation (45,155,156). Patient height and weight are positively and negatively, respectively, associated with the risk of endometriosis (157).
Endometriosis can be associated with significant gastrointestinal symptoms (pain, nausea, vomiting, early satiety, bloating and distention, altered bowel habits). A characteristic motility change (ampulla of Vater–duodenal spasm, a seizure equivalent of the enteric nervous system, along with bacterial overgrowth), is documented in most women with the disease (158). Women of reproductive age with endometriosis are not osteopenic (159).
The average delay between onset of pain symptoms and surgically confirmed endometriosis is quite long: 8 years or longer in the United Kingdom and 9 to 12 years in the United States (160). Similar durations were observed in Scandinavia and in Brazil (161,162). A delay in diagnosis of endometriosis of 6 and 3 years in women with pain and women with infertility, respectively, was reported. Over the past two decades, there was a steady decrease in the delay in diagnosis and a decline in the prevalence of advanced endometriosis at first diagnosis (163). Patient awareness of endometriosis was increased. Many patients’ quality of life is affected by pain, emotional impact of subfertility, anger about disease recurrence, and uncertainty about the future regarding repeated surgeries or long-term medical therapy and its side effects (164). Endometriosis should be perceived as a chronic disease, at least in a subset of highly symptomatic women, and quality-of-life issues should be evaluated using reliable and valid questionnaires (165).
Pain
In adult women, dysmenorrhea may be especially suggestive of endometriosis if it begins after years of pain-free menses. Dysmenorrhea often starts before the onset of menstrual bleeding and continues throughout the menstrual period. In adolescents, the pain may be present after menarche without an interval of pain-free menses. Evidence suggests that absenteeism from school and both the incidence and duration of oral contraceptive use for severe primary dysmenorrhea during adolescence is higher in women who later develop deeply infiltrative endometriosis than in women without deeply infiltrative endometriosis (166).
The distribution of pain is variable but most often is bilateral. Local symptoms can arise from rectal, ureteral, and bladder involvement, and lower back pain can occur. Some women with extensive disease have no pain, whereas others with only minimal to mild disease may experience severe pelvic pain. All endometriosis lesion types are associated with pelvic pain, including minimal to mild endometriosis (167). Endometriomas are not associated with dysmenorrheal severity, and dysmenorrhea is less frequent in women with only ovarian endometriomas compared with other locations (168,169). Endometriomas can be considered a marker for greater severity of deeply infiltrative lesions (170). Deeply infiltrative lesions are consistently associated with pelvic pain, gastrointestinal symptoms, and painful defecation (171). The role of adhesions in pain and endometriosis is poorly understood (172).
Many studies failed to detect a correlation between the degree of pelvic pain and the severity of endometriosis (11,169,173). Some studies reported a positive correlation between endometriosis stage and endometriosis-related dysmenorrhea or chronic pelvic pain (174,175). In one study, a significant but weak correlation was observed between endometriosis stage and severity of dysmenorrhea and nonmenstrual pain, whereas a strong association was found between posterior cul-de-sac lesions and dyspareunia (176).
Possible mechanisms causing pain in patients with endometriosis include local peritoneal inflammation, deep infiltration with tissue damage, adhesion formation, fibrotic thickening, and collection of shed menstrual blood in endometriotic implants, resulting in painful traction with the physiologic movement of tissues (177,178). The character of pelvic pain is related to the anatomic location of deeply infiltrating endometriotic lesions (171). Severe pelvic pain and dyspareunia may be associated with deep infiltrating subperitoneal endometriosis (6,177,179). In rectovaginal endometriotic nodules, a close histologic relationship was observed between nerves and endometriotic foci and between nerves and the fibrotic component of the nodule (180). Increasing evidence suggests a close relationship between the density of innervation of endometriotic lesions and pain symptoms (176).
Subfertility
Many arguments support the hypothesis that there is a causal relationship between the presence of endometriosis and subfertility (181). The following factors have been reported:
When endometriosis is moderate or severe, involving the ovaries and causing adhesions that block tubo-ovarian motility and ovum pickup, it is associated with subfertility (182,185). This effect was shown in primates, including cynomolgus monkeys and baboons (144,186). Numerous mechanisms (ovulatory dysfunction, luteal insufficiency, luteinized unruptured follicle syndrome, recurrent abortion, altered immunity, and intraperitoneal inflammation) are proposed as explanations, but an association between fertility and minimal or mild endometriosis remains controversial (187).
Spontaneous Abortion
A possible association between endometriosis and spontaneous abortion was suggested in uncontrolled or retrospective studies. Some controlled studies evaluating the association between endometriosis and spontaneous abortion have important methodologic shortcomings: heterogeneity between cases and controls, analysis of the abortion rate before the diagnosis of endometriosis, and selection bias of study and control groups (80,188,189). Based on controlled prospective studies, there is no evidence that endometriosis is associated with (recurrent) pregnancy loss or that medical or surgical treatment of endometriosis reduces the spontaneous abortion rate (190–192). Some data suggest that miscarriage rates may be increased after treatment with assisted reproductive technology (193).
Endocrinologic Abnormalities
Endometriosis is associated with anovulation, abnormal follicular development with impaired follicle growth, reduced circulating E2 levels during the preovulatory phase, disturbed luteinizing hormone (LH) surge patterns, premenstrual spotting, luteinized unruptured follicle syndrome, and galactorrhea and hyperprolactinemia (194). Increased incidence and recurrence of the luteinized unruptured follicle syndrome is reported in baboons with mild endometriosis, but not in primates with minimal endometriosis or a normal pelvis (195). Luteal insufficiency with reduced circulating E2 and progesterone levels, out-of-phase endometrial biopsies, and aberrant integrin expression was reported in the endometrium of women with endometriosis by some researchers, but these findings were not confirmed by other investigators (194,196,197). No convincing data exist to conclude that the incidence of these endocrine abnormalities is increased in women who have endometriosis.
Extrapelvic Endometriosis
Extrapelvic endometriosis, although often asymptomatic, should be suspected when symptoms of pain or a palpable mass occur outside the pelvis in a cyclic pattern. Endometriosis involving the intestinal tract (especially colon and rectum) is the most common site of extrapelvic disease and may cause abdominal and back pain, abdominal distention, cyclic rectal bleeding, constipation, and obstruction. Ureteral involvement can lead to obstruction and result in cyclic pain, dysuria, and hematuria. Pulmonary endometriosis can manifest as pneumothorax, hemothorax, or hemoptysis during menses. Umbilical endometriosis should be suspected when a patient has a palpable mass and cyclic pain in the umbilical area (56).
Treatment of extragenital endometriosis will depend on the site. If complete excision is possible, this is the treatment of choice; when this is not possible, long-term medical treatment is necessary using the same principles of medical treatment for pelvic endometriosis (1). Appendicular endometriosis is usually treated by appendectomy. Surgical treatment of bladder endometriosis is usually in the form of excision of the lesion and primary closure of the bladder wall. Ureteral lesions may be excised after stenting the ureter; in the presence of intrinsic lesions or significant obstruction, segmental excision with end-to-end anastomosis or reimplantation may be necessary. Abdominal wall and perineal endometriosis is usually treated by complete excision of the nodule (1).
Clinical Examination
In many women with endometriosis, no abnormality is detected during the clinical examination. However, the vulva, vagina, and cervix should be inspected for any signs of endometriosis, although the occurrence of endometriosis in these areas is rare (e.g., episiotomy scar). The presence of a narrow pinpoint cervical ostium can be a risk factor for endometriosis (23). Other signs of possible endometriosis include uterosacral or cul-de-sac nodularity, lateral or cervical displacement caused by uterosacral scarring, painful swelling of the rectovaginal septum, and unilateral ovarian cystic enlargement (198). In more advanced disease, the uterus is often in fixed retroversion, and the mobility of the ovaries and fallopian tubes is reduced. Evidence of deeply infiltrative endometriosis (deeper than 5 mm under the peritoneum) in the rectovaginal septum with cul-de-sac obliteration or cystic ovarian endometriosis should be suspected when there is clinical documentation of uterosacral nodularities during menses, especially if CA125 serum levels are higher than 35 IU/mL (199–201). In these cases, black-blue colored lesions can sometimes be observed in the vagina during speculum examination.
The clinical examination may have false-negative results. The diagnosis of endometriosis should be confirmed by visual inspection during laparoscopy and by histological confirmation of endometriosis in biopsied lesions.
Imaging
Ultrasound
Peritoneal endometriosis cannot be reliably visualized by imaging techniques. Compared to laparoscopy, transvaginal ultrasound has no value in diagnosing peritoneal endometriosis, but it is useful in making or excluding the diagnosis of an ovarian endometrioma (1,202). Either transvaginal or transrectal ultrasonography can be used with high sensitivity and specificity for the diagnosis of ovarian endometrioma (202–204). The typical ultrasound features of an endometriotic ovarian cyst in premenopausal women were described as “ground glass echogenicity of the cyst fluid, one to four locules and no solid parts” (205). Transvaginal ultrasound may have a role in the diagnosis of endometriosis nodules with a diameter of 1 cm involving the bladder or rectum, but this is dependent on the interest and experience of the ultrasonographer and the quality and resolution of the ultrasound equipment.
Local guidelines for the management of suspected ovarian malignancy should be followed in cases of ovarian endometrioma (1). Ultrasound scanning with or without serum CA125 testing is usually used to try to identify rare instances of ovarian cancer; however, CA125 levels are frequently elevated in the presence of endometriomas, so this approach is often not useful (1).
Other Imaging
Other imaging techniques, including computed tomography (CT) and MRI, can be used to provide additional and confirmatory information, but they cannot be used for primary diagnosis (1,206). These techniques are more costly than ultrasonography, and their added value is unclear.
Hysterosalpingography is not recommended as a diagnostic test for endometriosis, although the presence of filling defects (presence of hypertrophic or polypoid endometrium) has a significant positive correlation with endometriosis (positive and negative predictive values of 84% and 75%, respectively) (207).
Imaging to Assess Intestinal and Urologic Involvement
If there is clinical evidence of deeply infiltrating endometriosis, ureteral, bladder, and bowel involvement should be assessed. Ureteral involvement may be asymptomatic in up to 50% of patients with deeply infiltrative endometriosis (208). Consideration should be given to performing ultrasound (transrectal, transvaginal or renal), a CT urogram, or an MRI. A barium enema study might be useful, depending on the individual circumstances, to map the extent of disease present, which may be multifocal (1). There is no proof that one technique is superior to another; it is recommended that the technique that is most familiar to the radiologist involved be used.
Blood and Other Tests
There is no specific blood test for the diagnosis of endometriosis. A general endometriosis screening test may be neither appropriate (risk for overdiagnosis) nor feasible. A blood test with a high sensitivity would be useful if that would identify women with symptomatic endometriosis (pelvic pain, infertility) that is not detectable by ultrasound imaging (209). This would include all cases of minimal to mild endometriosis and those cases of moderate to severe endometriosis without detectable ovarian endometriotic cysts or nodules (210). These are patients who could benefit from laparoscopic surgery to reduce endometriosis-associated pain and infertility or to diagnose and treat other pelvic causes of pelvic pain or infertility, like pelvic adhesions. From that perspective, a lower specificity would be acceptable because the main goal of such a test would be to rule in all women with potential endometriosis or other pelvic disease who might benefit from surgery (211).
CA125
Levels of CA125, a glycoprotein from coelomic epithelium and common to most nonmucinous epithelial ovarian carcinomas, are significantly higher in women with moderate or severe endometriosis and normal in women with minimal or mild disease (212,213). It is presumed that endometriosis lesions produce peritoneal irritation and inflammation and this leads to an increased shedding of CA125 (213). During menstruation, an increase in CA125 levels was shown in women with and without endometriosis (214–218). Other studies did not find an increase during menses, or found an increase only with moderate to severe endometriosis (219–222). The levels of CA125 vary widely: in patients without endometriosis (8–22 U/mL in the nonmenstrual phase), in those with minimal to mild endometriosis (14–31 U/mL in the nonmenstrual phase), and in those with moderate to severe disease (13–95 U/mL in the nonmenstrual phase). Compared with laparoscopy, measurement of serum CA125 levels has no value as a diagnostic tool (223).
The specificity of CA125 is reported to be higher than 80% in most studies. This high level of specificity is achieved in selected women with infertility or pain, who are known to be at risk for endometriosis. The low level of sensitivity of CA125 (20% to 50% in most studies) poses limitations for the clinical use of this test for diagnosis of endometriosis. Theoretically, the sensitivity might increase during the menstrual period, when the increase in CA125 level is more pronounced in women who have endometriosis. Studies using cutoff levels of 35 U/mL or 85 U/mL did not find a significant improvement in sensitivity (221,222,224). A sensitivity of 66% was found when the CA125 level was determined during both the follicular phase and the menstrual phase in each patient and when the ratio of menstrual versus follicular values (> 1.5) was used instead of one CA125 level (222). Other studies reported that the value of CA125 in diagnosis of endometriosis is limited but higher for moderate to severe disease, especially if serum CA125 concentrations are measured during the midfollicular phase (223,225).
Serial CA125 determinations may be useful to predict the recurrence of endometriosis after therapy (226,227). CA125 levels decrease after combined medical and surgical therapy or during medical treatment of endometriosis with danazol, gonadotropin-releasing hormone (GnRH) analogues, or gestrinone, but not with medroxyprogesterone acetate (MPA) or placebo (228–230). Levels of CA125 are reported to increase to pretreatment levels as early as 3, 4, or 6 months after the cessation of therapy with danazol, GnRH analogues, or gestrinone (218,229–233). Posttreatment increases in CA125 levels are reported to correlate with endometriosis recurrence (217,227,234). Other studies did not substantiate a correlation between posttreatment CA125 levels and disease recurrence (228,231,235).
Other Tests
It is not possible to diagnose endometriosis in a noninvasive way based on the increased concentration of cytokines and growth factors in peripheral blood or on endometrial biopsy analysis (46,236).
Laparoscopy
General Considerations
Unless disease is visible in the vagina or elsewhere, laparoscopy is the standard technique for visual inspection of the pelvis and establishment of a definitive diagnosis (1). There is insufficient evidence to justify timing the laparoscopy at a specific time in the menstrual cycle, but it should not be performed during or within 3 months of hormonal treatment so as to avoid underdiagnosis (1). Laparoscopic recognition of endometriosis will vary with the experience of the surgeon, especially for subtle bowel, bladder, ureteral, and diaphragmatic lesions (1). A meta-analysis of its value against a histological diagnosis showed (assuming a 10% pretest probability of endometriosis) that a positive laparoscopy increases the likelihood of disease to 32% (95% confidence interval [CI], 21%–46%) and a negative laparoscopy decreases the likelihood to 0.7% (95% CI, 0.1%–5.0%) (1,237). Diagnostic laparoscopy is associated with an approximately 3% risk of minor complications (e.g., nausea, shoulder tip pain) and a risk of major complications (e.g., bowel perforation, vascular damage) of 0.6 to 1.8 per 1,000 cases (1,238,239). Endometriosis can be treated during laparoscopy, thus combining diagnosis and therapy.
Laparoscopic Technique
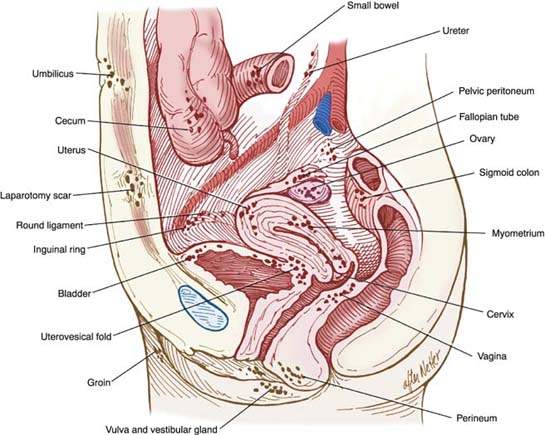
During diagnostic laparoscopy, the pelvic and abdominal cavity should be systematically investigated for the presence of endometriosis. This examination should include a complete inspection in a clockwise or counterclockwise fashion with a blunt probe, with palpation of lesions to check for nodularity as a sign of deeply infiltrative endometriosis of the bowel, bladder, uterus, tubes, ovaries, cul-de-sac, or broad ligament (Fig. 17.1). The type, location, and extent of all lesions and adhesions should be documented in the operative notes; ideally, the findings should be recorded with photographs or on video such as a DVD (1).
Figure 17.1 Pelvic endometriosis.