Fig. 6.1
Endometrial polyp as seen by hysteroscopy
6.2 Histological Features and Classification
The pathogenesis of endometrial polyps is not well understood, but they appear to originate from the endometrial basalis layer as focal overgrowths and consist of glands, stroma and thick-walled vessels (Mazur and Kurman 2005; Kurman 1982; Mutter et al. 2009). They may have a large flat base (sessile) or be attached to the endometrium by an elongated pedicle (pedunculated). Despite their diverse growth pattern, all endometrial polyps show typical histological features (Mazur and Kurman 2005; Kurman 1982; Mutter et al. 2009; Schlaen et al. 1988). In biopsy specimens, endometrial polyps typically present as larger, often polypoid shaped, tissue fragments with surface epithelium on three sides. The stroma of the polyp can be highly variable, but is often dense, and may include thick-walled vessels. The glands are irregular in shape and have a variable architecture.
Benign endometrial polyps can be classified, based on morphological features, into five morphological forms: proliferative/hyperplastic polyps, atrophic polyps, functional polyps, mixed endometrial–endocervical polyps, and adenomyomatous polyps (Mazur and Kurman 2005). The morphological patterns of benign endometrial polyps often overlap and have limited clinical significance for therapeutic decisions. Endometrial polyps are usually benign, but they may contain focal hyperplasia, focal complex hyperplasia, atypical hyperplasia, intraepithelial carcinoma or carcinoma (Peterson and Novak 1956; Mazur and Kurman 2005).
6.3 Prevalence
In textbooks of pathology, the prevalence of endometrial polyps is quoted to be approximately 25 % (Mazur and Kurman 2005; Kurman 1982; Mutter et al. 2009). The reported prevalence of endometrial polyps in women suffering from AUB varies between 10 and 40 % (Anastasiadis et al. 2000; Clevenger-Hoeft et al. 1999; Cohen 2004; Goldstein et al. 1997; Nagele et al. 1996a; van Bogaert 1988). The prevalence appears to increase by age during the reproductive years, but it is not clear whether it subsequently peaks or decreases after menopause (Anastasiadis et al. 2000; Clevenger-Hoeft et al. 1999; Peterson and Novak 1956; Reslova et al. 1999; van Bogaert 1988). Endometrial polyps are more common in women suffering from AUB, compared to women without such symptoms (Clevenger-Hoeft et al. 1999). Consequently, the large variation in reported prevalence of polyps may, among other factors, be explained by heterogeneous study populations in terms of age and eventual presence of symptoms. Furthermore, varying definitions and diagnostic methods, and difficulties in establishment of the histological diagnosis may contribute to the large variation in the reported prevalence of endometrial polyps (Mazur and Kurman 2005; van Bogaert 1988; Dreisler et al. 2009a).
The prevalence of endometrial polyps in asymptomatic women has been sparsely studied. In asymptomatic premenopausal women, the reported prevalence of endometrial polyps varies between 1 and 11 % (Clevenger-Hoeft et al. 1999; Dreisler et al. 2009a; Cooper et al. 1983; DeWaay et al. 2002). In asymptomatic women aged 45–50 years, the prevalence is reported to be 12 %, and in two studies evaluating asymptomatic postmenopausal women, the prevalence of endometrial polyps was found to be 12 and 17 % respectively (Dreisler et al. 2009a; Fay et al. 1999; Lieng et al. 2009).
6.4 Pathophysiology
Although endometrial polyps occur relatively often, the knowledge of the aetiology and pathogenesis of such polyps is limited. Endometrial polyps are believed to develop as a consequence of focal stromal and glandular overgrowth caused by prolonged oestrogen exposure (Schlaen et al. 1988; Lopes et al. 2007; Ryan et al. 2005; Sant’Ana de Almeida et al. 2004). The balance between mitotic activity and apoptosis is considered to regulate normal endometrial development during the menstrual cycle, and disturbances of these physiological processes have been proposed to occur in endometrial polyps (Stewart et al. 1999). Oestrogen and progesterone act, via their receptors, as modulators of proliferation and differentiation in the normal endometrium (Inceboz et al. 2006). Both oestrogen and progesterone receptors have been identified in the glandular epithelium of endometrial polyps in both post- and premenopausal women, but the receptor expression appears to be disorderly compared with normal endometrium (Lopes et al. 2007; Ryan et al. 2005; Mittal et al. 1996; McGurgan et al. 2006a).
Furthermore, demonstrations of increased levels of Ki61, a marker of cell proliferation, and Bcl-2, an inhibitor of apoptosis, indicate that loss of usual control mechanisms for growth may be of importance in development of endometrial polyps (Inceboz et al. 2006; McGurgan et al. 2006a; Maia et al. 2004a; Mertens et al. 2002; Taylor et al. 2003). This loss of proapoptotic mechanisms may be related to unopposed hyperestrogenism, because Bcl-2 expression increases in response to oestrogen (Mertens et al. 2002; Dahmoun et al. 1999). Oestrogen may consequently have a role in the development of endometrial polyps either by direct stimulation of localized proliferation, or by stimulation of proliferation via activation of Ki67 or inhibition of apoptosis via Bcl-2 (Inceboz et al. 2006). Available data also indicate that unopposed hyperestrogenism can lead to an abnormal increase of certain growth factors (GF) and growth factor receptors within the endometrium, which may stimulate endometrial polyp growth (Maia et al. 2001). Cytogenic abnormalities following altered gene expressions have been identified in endometrial polyp tissue (Bol et al. 1996; Nogueira et al. 2006; Vanni et al. 1993). However, any effect of these findings in the development of endometrial polyps is not known, and the studies are small and need to be confirmed in future studies including a larger number of endometrial polyps.
6.5 Associated Factors
Increasing age appears to be the best-documented risk indicator for endometrial polyps (Anastasiadis et al. 2000; Clevenger-Hoeft et al. 1999; Reslova et al. 1999; Nagele et al. 1996a; van Bogaert 1988; Dreisler et al. 2009a; Nappi et al. 2009; Vilodre et al. 1997). Some authors report that the peak prevalence of endometrial polyps occurs during the last decade of the fertile years (Clevenger-Hoeft et al. 1999; van Bogaert 1988), while others report menopause as a risk indicator for the development of endometrial polyps (Anastasiadis et al. 2000; Reslova et al. 1999; Nagele et al. 1996a; Dreisler et al. 2009a; Vilodre et al. 1997). In two reports using age-adjusted regression models, menopause was not found to be associated with a higher prevalence of endometrial polyps (Nappi et al. 2009; Dreisler et al. 2009b).
Endometrial polyps have been reported to occur more often in women with obesity, hypertension, fibroids, endometriosis, or cervical polyps (Clevenger-Hoeft et al. 1999; Peterson and Novak 1956; Reslova et al. 1999; Vilodre et al. 1997; Dreisler et al. 2009b; Onalan et al. 2009; Oguz et al. 2005; Coeman et al. 1993; Kim et al. 2003; McBean et al. 1996). The results of two prospective trials including 245 and 375 women, respectively, indicate an association of obesity and endometrial polyps (Reslova et al. 1999; Oguz et al. 2005). This association was also reported in a retrospective study including 230 women undergoing in vitro fertilization (IVF), where BMI ≥ 30 kg/m2 was found to be an independent risk factor for the development of endometrial polyps (Onalan et al. 2009). Obesity is characterized by decreased sex-hormone-binding globulin (SHBG) levels, increased aromatization of androgens to oestrogens in adipose tissue and high levels of unopposed oestrogen in the circulation (Onalan et al. 2009). This relative hyperestrogenism may explain the development of endometrial polyps in obese women. Both obesity and hypertension are conditions related to an abnormal increase in serum and endometrial tissue of growth factors such as free insulin-like GF (IGF)-1, which might be of importance for the development of endometrial polyps (Maia et al. 2001). But reports regarding obesity as an associated factor with endometrial polyps are not consistent.
Similarly, a relationship between hypertension and development of endometrial polyps has been suggested (Reslova et al. 1999). However, no association between hypertension and development of endometrial polyps was found in the two studies using logistic regression models for adjustments for age and overweight (Nappi et al. 2009; Dreisler et al. 2009b).
Endometrial polyps are furthermore reported to occur more often in women with fibroids, cervical polyps, and endometriosis (Clevenger-Hoeft et al. 1999; Peterson and Novak 1956; Vilodre et al. 1997; Coeman et al. 1993; Kim et al. 2003; McBean et al. 1996). Most of these studies are retrospective and include relatively few patients. The pathogeneses of any associations between endometrial polyps and fibroids, cervical polyps and endometriosis appears not to be considered, but it might be related to oestrogen influence.
Tibolon appears to increase the risk of endometrial polyp development (Perez-Medina et al. 2003). Data regarding an eventual relationship between hormone therapy (HT) and endometrial polyps are contradicting, as some studies report higher prevalence of endometrial polyps in women using HT (Dreisler et al. 2009b; Maia et al. 1996), whereas others do not (Akkad et al. 1995; Bakour et al. 2002; Elliott et al. 2003; Orvieto et al. 1999). In an experimental study, Maja et al. found that HT may cause endometrial polyp involution by decreasing proliferation and stimulating apoptosis (Maia et al. 2004b). In accordance with this finding, Perrone et al. reported a lower prevalence of endometrial polyps in women using HT compared to a control group (Perrone et al. 2002). Endometrial polyp formation has been reported to be dependent on the type and dosage of HT (Oguz et al. 2005; Iatrakis et al. 2006). Furthermore, a progestogen with high antipestrogenic activity, as well as use of oral contraceptive pills may have a protective effect on the development of endometrial polyps (Dreisler et al. 2009b; Oguz et al. 2005). An eventual protective effect of levonorgesterel-releasing intrauterine devices on the development of endometrial polyps seems not to have been evaluated.
Tamoxifen has in previous studies shown a consistent relationship with the development of endometrial polyps (Reslova et al. 1999; Cohen 2004; Bakour et al. 2002; Chalas et al. 2005; De Muylder et al. 1991; Kedar et al. 1994). Tamoxifen appears to have a significant effect on hormone receptor expression and markers of apoptosis in endometrial polyps, which support the hypothesis stating that tamoxifen promotes polyp growth by inhibiting apoptosis (McGurgan et al. 2006b). This hypothesis is furthermore substantiated by the report by Gardener et al., indicating that the levonorgestrel-releasing intrauterine system has a protective action against the uterine effects of tamoxifen (Gardner et al. 2000).
6.6 Symptoms and Clinical Consequences
The majority of endometrial polyps are probably asymptomatic (Ryan et al. 2005). Most symptomatic women with endometrial polyps present with different forms of AUB (intermenstrual bleeding, menorrhagia, metrorrhagia, postmenopausal bleeding), and endometrial polyps are found in about 10–40 % of women suffering from such symptoms (Anastasiadis et al. 2000; Clevenger-Hoeft et al. 1999; Goldstein et al. 1997; van Bogaert 1988). It is not known why endometrial polyps may cause AUB, but it is believed that abbreviations in hormone receptor expression within polyps may lead to an abnormal response to the hormonal environment, causing tissue breakdown and bleeding (Ryan et al. 2005). Although different forms of AUB constitute the dominating symptom in women with endometrial polyps, women with such polyps also may present with dysmenorrhoea, vaginal discharge or endometritis caused by extension of large polyps into the endocervix by dilatation of the internal cervical os (Mazur and Kurman 2005; Ryan et al. 2005).
In a large prospective trial evaluating 1,000 infertile women scheduled for IVF, the prevalence of endometrial polyps was found to be 32 % (Hinckley and Milki 2004). The high prevalence of endometrial polyps in infertile women suggests a causative relationship between the presence of endometrial polyps and infertility (Hinckley and Milki 2004; Frydman et al. 1987; Seinera et al. 1988; Syrop and Sahakian 1992). However, a causal relationship between endometrial polyps and infertility appears to have been confirmed in only one prospective randomized trial (Perez-Medina et al. 2005). In this trial allocating women with endometrial polyps to hysteroscopic polypectomy or diagnostic hysteroscopy prior to intrauterine insemination, women in the resection group had a significant higher chance of becoming pregnant. The results of a retrospective trial also indicate that hysteroscopic polypectomy may enhance fertility in women with endometrial polyps compared with infertile women with normal cavity (Varasteh et al. 1999). On the other hand, endometrial polyps did not appear to impair implantation outcome during IVF in two other retrospective studies (Isikoglu et al. 2006; Lass et al. 1999). A trend towards increased early pregnancy loss among women with endometrial polyps was reported in one of these studies (Lass et al. 1999).
The knowledge regarding the natural history and clinical consequences of endometrial polyps without treatment is limited. Only two studies have prospectively followed a cohort of women with endometrial polyps to investigate the spontaneous polyp regression rate (DeWaay et al. 2002; Lieng et al. 2009). The follow up-period was 2.5 and 1 year, and the spontaneous regression rate was reported to be 0.6 and 0.3, respectively. In both studies, polyps that regressed tended to be smaller compared to polyps that persisted during the follow-up period. Because of small study samples, these results must be interpreted with care.
6.7 Malignancy
Although the knowledge regarding the malignant potential of endometrial polyps is limited, polyps are believed to be a risk indicator for the development of premalignant and malignant tissue changes (Savelli et al. 2003). Both atypical hyperplasia and endometrial carcinoma may originate from endometrial polyps. The most common subtypes of endometrial carcinoma in malignant endometrial polyps appear to be endometroid carcinoma and serous papillary carcinoma (Farrell et al. 2005; Giordano et al. 2007). Most commonly, endometrial carcinoma arising in endometrial polyps is an early carcinoma with good prognosis, except for papillary serous carcinoma, which can be associated with omental involvement, despite low stage of malignant development in the uterus (Giordano et al. 2007).
There appears to be only one controlled study evaluating an association of endometrial polyps with premalignant and malignant pathology (Bakour et al. 2000). In this prospective study including 248 women with AUB, hyperplasia was found to be more frequent among women with endometrial polyps compared to women without polyps (7/62 vs 8/186), but no such differences between the two study groups were found for malignancy (2/62 vs 6/162).
The results of numerous previous case series evaluating histological diagnosis of resected polyps indicate that atypia and malignancy occur within 0.3–23.8 % and 0.8–3.2 % of endometrial polyps respectively (Anastasiadis et al. 2000; Orvieto et al. 1999; Savelli et al. 2003; Bakour et al. 2000; Antunes et al. 2007; Ben-Arie et al. 2004; Ferrazzi et al. 2009; Machtinger et al. 2005; Papadia et al. 2007; Shushan et al. 2004; Lieng et al. 2007). Most authors seem to agree that the prevalence of malignancy in endometrial polyps varies by age and menopausal status. According to the available evidence, the risk of malignancy in premenopausal women appears to be low. Gynaecological symptoms have also been identified as a possible risk indicator of malignancy within endometrial polyps (Antunes et al. 2007; Ferrazzi et al. 2009; Shushan et al. 2004; Lieng et al. 2007). However, previous reports evaluating the prevalence of malignancy within endometrial polyps in symptomatic as well as asymptomatic women are not consistent. Polyp size has been reported to be a risk indicator for malignant endometrial polyps (Ben-Arie et al. 2004; Ferrazzi et al. 2009), among others by the authors of a large retrospective multicenter study including both asymptomatic and symptomatic postmenopausal women with endometrial polyps (Ferrazzi et al. 2009).
Although the reports are not consistent, other known risk factors for endometrial carcinoma, such as obesity, diabetes mellitus and hypertension have also been reported to increase the risk of malignancy within endometrial polyps (Anastasiadis et al. 2000; Savelli et al. 2003; Bernstein et al. 1999). Furthermore, tamoxifen appears to increase the risk of atypical hyperplasia and malignancy in endometrial polyps (Cohen 2004; Kedar et al. 1994; Bernstein et al. 1999).
The reported prevalence of atypia and malignancy within endometrial polyps consequently varies according to the population studied, and the relatively large variation in reported prevalence probably reflects that the populations in the different studies are heterogeneous in terms of age and symptoms. Furthermore, the definition of a malignant endometrial polyp varies in previous publications, as some define the malignant tissue changes as a malignant polyp only if the stalk and surrounding endometrium are free of cancer, while others do not require this as an assumption (Ferrazzi et al. 2009). Varying definitions of the histopathological diagnosis may consequently contribute to the relatively large variation of reported prevalence of malignancy within endometrial polyps.
6.8 Diagnosis
Previously, endometrial polyps were diagnosed by histological examination of specimens retrieved by curettage and hysterectomy from women suffering from AUB. The technical advancement of ultrasound, including high-frequency vaginal transducers, has enabled an improved view of the endometrium. This has led to a new era in the diagnostics of focal intracavitary processes.
In women with postmenopausal bleeding, measurements of the bilayer endometrial thickness have been documented effective for exclusion of malignancy, but it appear to have poor discriminative ability for detecting or excluding endometrial polyps (Gupta et al. 2002; Timmermans et al. 2008). In premenopausal women with AUB, diverging results have been reported regarding the diagnostic value of endometrial thickness measurements for the detection of focal intracavitary processes, including endometrial polyps (Goldstein et al. 1997; Dijkhuizen et al. 1996; Dueholm et al. 2001; Schwarzler et al. 1998; Vercellini et al. 1997). Despite the widespread use of TVUS during general gynaecological examinations, the diagnostic value of endometrial thickness measurements in asymptomatic women has been sparsely investigated. In a study including 375 asymptomatic women aged 20–74 years, measurement of endometrial thickness by TVUS was found of little use as a diagnostic tool for the detection of focal intracavitary processes, but more efficacious in excluding focal intrauterine pathology, especially in postmenopausal women (Dreisler et al. 2009c). Sonographic examination using artificial uterine cavity distension, such as saline infusion sonography (SIS) or gel installation sonography (GIS), appears to improve the diagnostic accuracy of TVUS in case of abnormal or inclusive findings and has a high sensitivity and specificity for the detection of intrauterine pathology (Fig. 6.2) (Anastasiadis et al. 2000; Syrop and Sahakian 1992; van Roessel et al. 1987; Parsons and Lense 1993; de Kroon et al. 2003; Jansen et al. 2006; Guven et al. 2004). Hysteroscopy and SIS appear to be equivalent diagnostic tools for the detection of endometrial polyps and intrauterine myomas in the evaluation of both premenopausal and postmenopausal women with AUB (van Roessel et al. 1987; Parsons and Lense 1993; Dijkhuizen et al. 2000; Pasqualotto et al. 2000; Widrich et al. 1996).
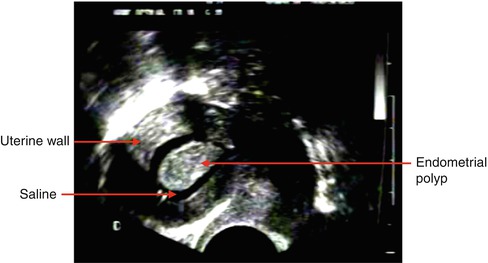

Fig. 6.2
Endometrial polyp protruding into the uterine cavity as seen during saline infusion sonography
Demonstration of the feeding vessel of the endometrial polyp by transvaginal colour or power Doppler examination may be of diagnostic importance in discrimination of endometrial lesions with different vascular patterns, such as polyps and fibroids (Fig. 6.3) (Krampl et al. 2001; Exalto et al. 2007; Fleischer and Shappell 2003).
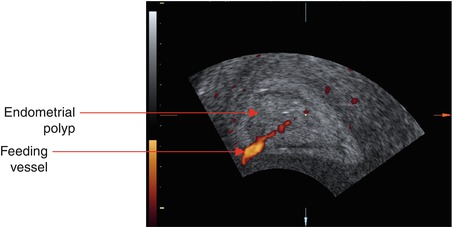

Fig. 6.3
Power Doppler sonogram showing the feeding vessel characteristic of endometrial polyps
Neither ultrasonography nor hysteroscopic features have been proved successful in distinguishing benign from malignant endometrial polyps (Shushan et al. 2004; Jakab et al. 2005; Timmerman et al. 2003; Bettocchi et al. 2004a). Previous studies suggest that evaluation of tissue vascularization by power or colour flow Doppler imaging may be useful in the prediction of malignant endometrial changes, due to the increased blood flow in malignant lesions (Epstein et al. 2001; Fernandez-Parra et al. 2006). Low Doppler resistance of the feeding vessel of endometrial polyps has been reported to be predictive of atypia and malignancy (Aleem et al. 1995; Lieng et al. 2008).
Techniques such as three-dimensional (3D) ultrasonography, power Doppler angiography and contrast-enhanced ultrasonography (CEUS) appear to improve the quality and accuracy of Doppler examinations and provide a relatively new and more comprehensive assessment of tumour vascularization, compared to the two-dimensional colour or power Doppler. Such new techniques may prove to be useful for prediction of atypia or malignancy in intrauterine lesions such as endometrial polyps, in the future. However, this needs further investigation.
Accordingly, histopathology is still required to exclude atypia and malignancy within endometrial polyps. The diagnostic accuracy of blind endometrial sampling obtained by an endometrial suction curette is low in the presence of endometrial polyps (Maia et al. 1996; Carter et al. 1994; Perez-Medina et al. 2002; Merce et al. 2007; Raine-Fenning et al. 2003). A device for SIS-based guided biopsies has been developed, but the diagnostic accuracy of this method has not been evaluated in comparative studies (Sidhu et al. 2006). As curettage misses the endometrial polyps in many cases, hysteroscopy combined with histopathological examination of retrieved specimens is still the gold standard for exclusion of malignancy in endometrial polyps (Bokor et al. 2001; Testa et al. 2005).
6.9 Treatment
Treatment of endometrial polyps is performed in order to exclude premalignant and/or malignant tissue changes, relieve symptoms (AUB) or improve fertility outcomes in infertile women.
Endometrial polyps were previously treated by curettage or hysterectomy (Elpek et al. 1998). The results of previous studies indicate that about 10 % of polyps remain in situ after curettage (Hann et al. 2003; Svirsky et al. 2008). More recently, Bonvolonta et al. found, by control hysteroscopy after curettage, that the polyp was fully removed in only 2 out of 25 women (Tanriverdi et al. 2004). As opposed to curettage, hysteroscopy allows, under visional control, the complete removal of the polyp. Due to the considerable limitation of curettage, transcervical (hysteroscopic) resection of endometrial polyps (TCRP) is today regarded as the optimal treatment of endometrial polyps (de Kroon and Jansen 2006; Smith and Schulman 1985; Gimpelson and Rappold 1988). The procedure is most often performed in an inpatient setting under general anaesthesia (Chavez et al. 2002). However, it appears that at least smaller endometrial polyps may be resected in an office setting without significant discomfort to the women (Englund et al. 1957).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


