Chapter 5 Endocrinology of Pregnancy and Parturition
 Hormones
Hormones
PEPTIDE HORMONES
STEROID HORMONES
Progesterone
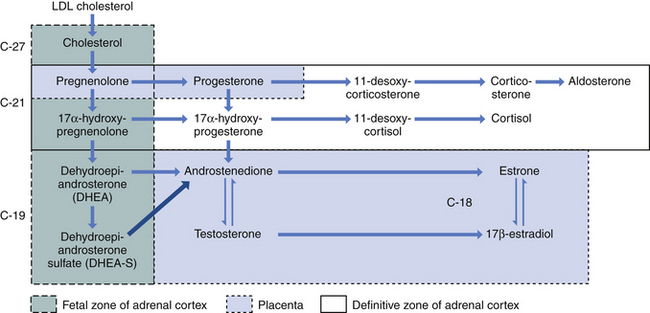
The fetus inactivates progesterone by transformation to corticosteroids or by hydroxylation or conjugation to inert excretory products. However, the placenta can convert these inert materials back to progesterone. Steroid biochemical pathways are shown in Figure 5-1.
Estrogens
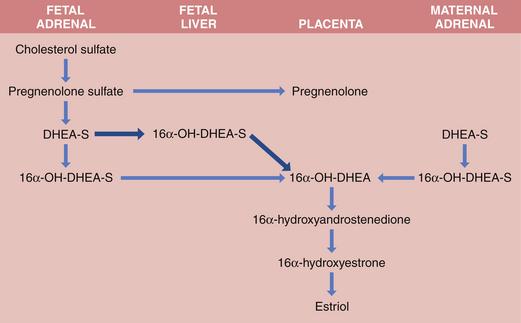
Both fetus and placenta are involved in the biosynthesis of estrone, estradiol, and estriol. Cholesterol is converted to pregnenolone and pregnenolone sulfate in the placenta. These precursors are converted to DHEA-S largely in the fetal, and to a lesser extent the maternal, adrenals. The DHEA-S is further metabolized by the placenta to estrone (E1) and, through testosterone, to estradiol (E2). Estriol (E3), the most abundant estrogen in human pregnancy, is synthesized in the placenta from 16α-hydroxy-DHEA-S, which is produced in the fetal liver from adrenal DHEA-S. Placental sulfatase is required to deconjugate 16α-hydroxy-DHEA-S before conversion to E3 (Figure 5-2). Steroid sulfatase activity in the placenta is high except in rare cases of sulfatase deficiency.
 Fetoplacental Unit
Fetoplacental Unit