Hormones of Pregnancy
• hCG is secreted by placental trophoblast. Detected in the mother’s bld 8 d after conception. Maintains corpus luteum progesterone production. Structurally similar to LH, FSH, & TSH (same alpha-subunit). Doubles q48h early Preg. Max 8–10 w, ∼100000 mIU/mL → decline at 10–12 w → nadir at 20 w.
• hPL is produced by syncytiotrophoblasts. Detected at 2–3 w after fertilization. Levels rise steadily until 34–36 w to a peak 5–10 μg/mL. Effects include mat lipolysis → ↑ circulating free fatty acids to provide a source of energy for mother & fetus; anti-insulin action → increased mat insulin levels → increased prot synthesis; angiogenic action → fetal vasculature formation
• Progesterone is mainly produced by the ovary until 6–7 w gest when the placenta begins to produce. Maintains endometrial lining in early Preg & uterine quiescence. Production ∼250–600 mg/d (prepregnancy 0.1–40 mg/d).
• Relaxin is secreted by the corpus luteum → uterine relaxation, systemic vasodilation, & ↑ cardiac output. Serum concentrations peak at 1 ng/mL at 12–13 w → fall to 0.5 ng/mL for the remainder of the Preg (Am J Physiol Regul Integr Comp Physiol 2011:R267).
TYPE I DIABETES MELLITUS
Definition and Epidemiology (Diabetes Care 2012;35(suppl 1):S64)
• Gluc intolerance due to insulin insufficiency. Often caused by cell-mediated autoimmune Pancr β-cell destruction. Only about 5% of all diabetes.
• Incid increasing 2–5%. Prevalence 1 in 300 by age 18 (Endocrinol Metab Clin North Am 2010;3:481)
• A/w other autoimmune diseases (eg, Graves, Hashimoto, Addison dz) (Diabet Med 2011;28(8):896)
Etiology and Pathophysiology
• Genetic: 95% have either HLA-DR3 or HLA-DR4. Also positive for anti-GAD, anti-islet cell, & anti-insulin Abs.
• Environmental: Congen rubella infxn, enterovirus, coxsackievirus B, CMV, adenovirus, & mumps (Diabetes Care 2012(suppl 1):S64)
• Lymphocytic infiltration, β-cell ↓ → insulin deficiency (Diabetes Metab Res Rev 2011;8:778)
• Hyperglycemia at ∼80–90% β-cell loss
Clinical Manifestations
• Polyuria, polydipsia, polyphagia w/ weight loss, fatigue, weakness, muscle cramps, blurred vision, nausea, abdominal pain, changes in bowel mvmt
• Most present w/ acute sx of diabetes & markedly elevated bld gluc levels
Diagnostic Workup
• Screen high-risk individuals (h/o transient hyperglycemia or relative w/ type I DM)
• Islet auto Abs ↑ risk of developing type I DM. Criteria for DM same for type I or II (below).
Treatment and Medications (JAMA 2003;289(17):2254)
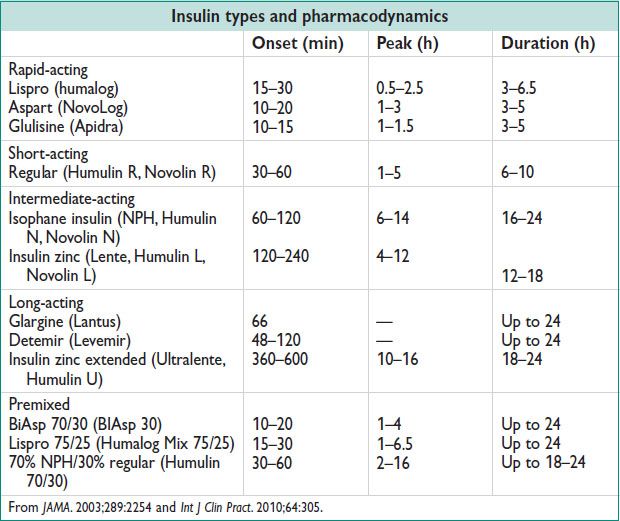
• Lifelong insulin therapy is started w/ either MDI therapy, or CSII. See insulin types, below.
• Gluc measurements can be done either preprandial only, or pre- & postprandial (shows greater improv in glycemic control) (Clin Med 2011;2:154)
• MDI nonphysiologic regimens – do not mimic nml insulin secretion
Once daily long-acting insulin (at bedtime)
Twice daily intermediate-acting insulin (breakfast & dinner time)
• MDI physiologic regimens – attempt to mimic nml insulin secretion
Twice daily intermediate-acting insulin w/ short-acting insulin (breakfast & dinner time)
Once daily long-acting insulin (at bedtime) w/ mealtime rapid-acting insulin
Twice daily intermediate-acting insulin (breakfast & bedtime) w/ rapid acting insulin w/ each meal
Premixed insulin (70% NPH/30% regular) given twice daily
• CSII – Rapid-acting insulin preparation administered through a catheter that is inserted into the SQ tissue. There is a basal insulin infusion rate (1 U/h) w/ patient-directed boluses given before meals.
• Nonpregnant goal: HgA1c <7%, fasting gluc 70–130 mg/dL, postprandial gluc <180 mg/dL
DIABETIC KETOACIDOSIS (DKA)
Definition
• An acute life-threatening complication due to insulin deficiency, w/ hyperglycemia, dehyd, & acidosis. Typically due to insulin noncompliance, acute illness/infxn, drugs, or new onset DM.
• Occurs in 5–10% of all pregnancies w/ DM. Can develop more rapidly & at less sev levels of hyperglycemia than in nonpregnant pts.
Pathophysiology (Clin Med 2011;2:154)
• Insulin deficiency → ↑ glucagon → ↑ hepatic gluconeogenesis & ↑ glycogenolysis → hyperglycemia → inability to use gluc → ↑ lipolysis → free fatty acids metabolized by liver (ketogenesis) as an alternative energy source → large quantities of ketones → acidosis
Clinical Manifestation (Hormones 2011;4:250)
• Nausea, vomiting, abdominal pain, confusion, Kussmaul respirations (deep labored breathing seen in metabolic acidosis).
Diagnostic Workup
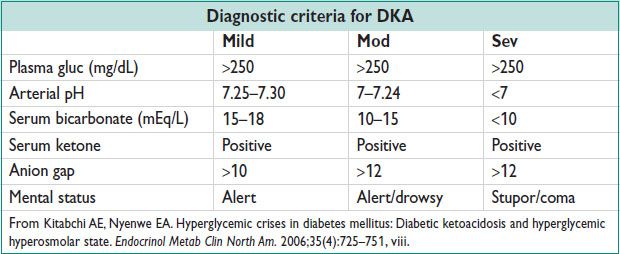
• Bld gluc, bld gas (pH), Chemistry (bicarbonate, anion gap), serum ketones
Treatment
• Treat the underlying cause (eg, infxn). Inpatient admission.
• Fluids: 1 L NS 1st hour, then 250–500 mL/h. When gluc <250 mg/dL → change to 5% dextrose in ½ NS, and continue insulin till ketonemia resolved.
• Insulin: 0.1–0.4 U/kg IV bolus → 0.1 U/kg/h continuous infusion (or 2–10 U/h). Try for 50–70 mg/dL/h correction of serum gluc, or about 25% in 1st 2 h. When plasma gluc is ∼200 mg/dL → ↓ insulin to 0.05 U/kg/h (or about 1–2 U/h) until urine ketones cleared. Adjust till gluc ∼150–200 mg/dL. When pt can tolerate food, start her usual SQ insulin injection regimen.
• Potassium: K >5 mEq/L, no additional req. (Insulin drives K into cells w/ gluc → ↓ serum K.)
K 4–5 mEq/L → add 20 mEq/L to each liter of replacement fluid
K 3–4 mEq/L → add 40 mEq/L to each liter of replacement fluid
K <3 mEq/L → hold insulin, give 10–20 mEq/h until K >3.3, then 40 mEq/L in IVF
• Bicarbonate: pH <6.9 → give 100 mEq & 20 mEq of KCl in 400 mL of H2O over 2 h
pH <7 or bicarbonate <5 mEq → give 50 mEq in 200 mL of water over 1 h until ph ↑ to >7
Do not give bicarbonate for pH >7
• Phosphate: If <1 mg/dL → give 20–30 mmol potassium phosphate over 24 h
• Calcium: Monit serum Ca level & replete prn
• Fetal HR monitoring for >24 w gest. Fetal loss 9–85% depending on severity of DKA.
TYPE II DIABETES MELLITUS
Definition and Epidemiology (Diabetes Care 2012;35(suppl 1):S64)
• Insulin resistance ± inadeq insulin production (ie, inadeq production for the sens of the target tissues). ∼26 million people w/ DM, ∼79 million prediabetes, & 1.9 million new cases of DM diagnosed in 2010 (National Diabetes Fact Sheet, www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf)
Pathophysiology
• Periph insulin resistance → ↑ insulin secretion → Pancr failure → defective insulin secretion in resp to ↑ gluc → increased liver gluconeogenesis → hyperglycemia
Clinical Manifestation
• Classical sx: Polyuria, polydypsia, polyphagia, fatigue, weakness, muscle cramps, blurred vision, nausea, abdominal pain, changes in bowel mvmt. Most are asx.
Diagnostic Workup
• Criteria for diagnosing T2DM outside of Preg: Hgb A1c ≥6.5%, fasting gluc ≥126 mg/dL, 2-h 75 g OGTT plasma gluc ≥200 mg/dL, or a random plasma gluc ≥200 mg/dL in a pt w/ classic sx or in hyperglycemic crisis
Treatment and Medications
• Goal of rx is to achieve & maintain HbA1c levels of <7%. See also for Preg, below.
• At dx: Lifestyle changes (weight loss, exercise) may ↓ HbA1c 1–2%
• Bariatric Surg consideration for adults w/ T2DM & BMI >35 kg/m2
• See appendix for oral hypoglycemic agent meds. See Ch. 1 for well-woman mgmt.
HYPEROSMOLAR HYPERGLYCEMIC STATE
Etiology and Pathophysiology (Emerg Med Clin North Am 2005;23:629)
• Extreme hyperglycemia + hyperosmolality, w/o ketoacidosis
• Infxn causing about 60% of cases → physiologic stress → ↓ effectiveness of circulating insulin → ↑ counter regulatory hormones (glucagon, catecholamines, cortisol, GH) → ↑ periph resistance → gluconeogenesis → hyperglycemia → glycosuria → hypertonic osmotic diuresis (dehyd) → unable to maintain adequate fluid intake (2/2 acute illness) → sev hyperosmolality & intracellular dehyd, renal failure
Diagnostic Workup
• Plasma gluc level of ≥600 mg/dL, serum osmolality of ≥320 mOsm/kg, ↑ serum urea nitrogen (BUN): Cr ratio, pH >7.3, small ketonuria, absent to low ketonemia, bicarbonate >15 mEq/L
Treatment
• Treat the underlying cause. Mgmt very similar to DKA (above).
• 1st-line therapy is aggressive IV hydration, fluid deficit may be 8–12 L. Replace 1/2 of the fluid deficit in the 1st 12 h, & the remainder in the next 12–24 h w/ NS.
• Insulin infusion when potassium is ≥3.3 mEq/L. Regular insulin started at 0.1 U/kg/h w/ or w/o a 0.15 U/kg bolus.
• Once the serum gluc ≤300 mg/dL, D5 should be added & the insulin infusion ↓ to 0.05 U/kg/h
• If serum potassium level is <3.3 mEq/L → replete w/ KCl at a rate of up to 40 mEq/L/h until levels are above 3.3 mEq/L. 20 mEq/L KCl can then be added to each 1 L of IV fluid. Goal is to maintain nml serum K levels. Check K every 1–2 h.
DIABETES IN PREGNANCY
Epidemiology
• Pregestational diabetes in ∼1% of all pregnancies, mostly type II.
• 90% of diabetes in Preg is GDM (see GDM, below)
Clinical Manifestation
• Type I usually known prior to Preg. Type II may have been unrecognized, but if gluc intolerance before 20 w, consider pregestational. Goal preconception HgA1c <6.5%. Consider hospital admission for very poor control during organogenesis.
• Fetal malformation rate in a nml Preg is 2–3% vs. 6–12% in pregnancies c/b diabetes (Obstet Gynecol 2003;102:857). Rate of fetal malformations w/ Hgb A1c 7–8.9 = 5–10%; Hbg A1c 9–10.9 = 10–20%, HbgA1c >11 = >20%
• “Usual” defects include cardiac, renal, neural tube. Esp double outlet RV, truncus arteriosus, & caudal regression syn/sacral agenesis (considered pathognomonic).
• Risks of DM in Preg: ↑ malformations, ↑ SAB, ↑ IUGR, ↑ progression of nephropathy, retinopathy, cardiovascular dz, ↑ polyhydramnios, ↑ preeclampsia, ↑ labor dystocia & C/S deliv, ↑ fetal macrosomia, ↑ lacerations, ↑ shoulder dystocia, ↑ neonat RDS/hypoglycemia.
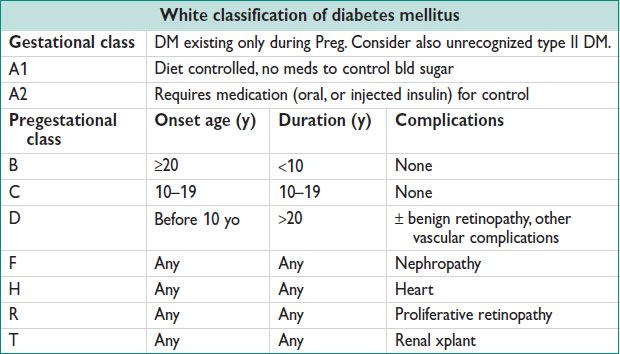
Screening for DM in Pregnancy
• Univ GDM screening std. Screen early if risk factors. Consider no screening by criteria.
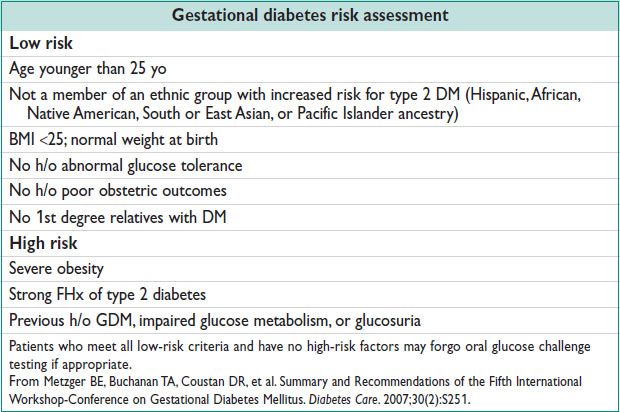
• On 50 g oral gluc challenge test, serum gluc ≥140 mg/dL identifies 80% GDM; ≥130 mg/dL identifies 90% GDM. Serum gluc ≥200 mg/dL → GDM w/o other testing. Positive screening test → 3 h fasting gluc challenge (100 g test; diagnostic table, below).
• 3-h OGTT: Consume ≥150 g of carbohydrate per day for 3 d, then fasting. 100 g oral gluc challenge → fasting + 1-, 2-, 3-h post challenge bld gluc. 1 abn value = gluc intolerance (a/w fetal macrosomia). Dx of GDM made ≥2 abn values.
• New Endo one step Guideline differs from ACOG (J Clin Endo Metab 2013;98:4227)
Universal DM testing before 13w gest, repeat if abnormal on different day to confirm. 8–14hr Fasting gluc ≥126 mg/dL, untimed ≥200mg/dL, or HbA1C ≥6.5% = overt DM; Fasting 92-125 mg/dL = GDM
24-28w screen if not prev dx, w/ 75g OGTT (after 8hr fast)
Fasting gluc >126mg/dL or 2hr >200 mg/dL = overt DM;
Fasting 92–125 mg/dL or 1hr >180 mg/dL or 2hr 153–199 mg/dL = GDM
Management of DM in Pregnancy
• GDM
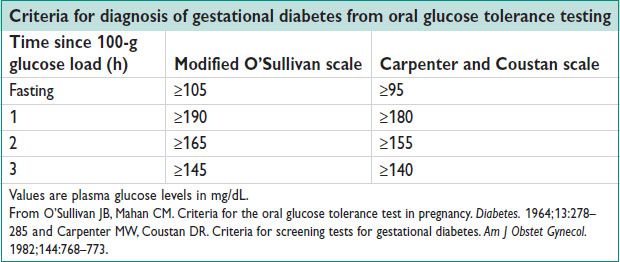
Nutrition advice, diet/exercise, & 4×/d bld gluc testing (fasting + 1- or 2-h postprandial)
If inadeq control → oral hypoglycemic agents (glyburide), if inadeq w/ max dose → insulin
GDM-A1 no monitoring, no early deliv (routine induction at 41–42 or for OB indications)
GDM-A2 antenatal testing (NST/BPP from 32–34 w) & deliver by 40 w
• Pregestational diabetes
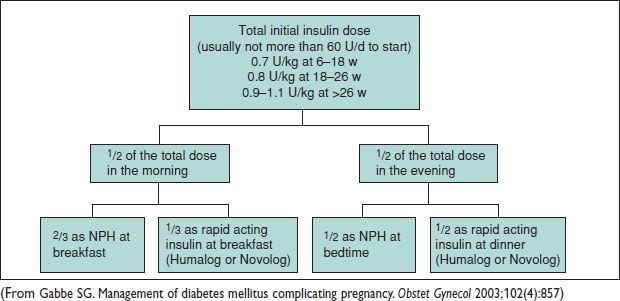
Diet: 1800–2400 kcal daily, w/ 20% prot, 60% carbs, & 20% fat. The American Diabetes Association recommends insulin for pregnant women w/ type I or II DM. NPH & rapid-acting insulin combination used (see Table with insulin types, above). Type I DM usually ↑ insulin 50–100%. Type II DM often ↑ >200% in Preg. Consider baseline HELLP labs, thyroid testing (40% type I DM – thyroid d/o) & 24-h urine prot early Preg.
Eye exam in 1st trimester, & baseline ECG (age >30 y or hypertensive).
Pregestational DM obtain early sonogram, confirm viability, offer mat serum AFP for NT defects, US for anatomy & fetal echocardiography.
1–2×/w fetal NST/AFI from 32–34 w or earlier. Serial fetal growth scans every 4–6 w to eval for IUGR or macrosomia. Deliv not later than 39–40 w, depending on gluc control in Preg.
Figure 17.2 Calculation and dose distribution for initial insulin management in pregnancy
Labor and Delivery for Diabetics
• Consider cesarean deliv for EFW >4500 g for pts w/ diabetes (>5000 g for nondiabetic)
• Insulin mgmt during labor: Usual intermediate insulin at bedtime. Morning dose insulin withheld. W/ active labor or gluc <70 mg/dL start D5NS IVF. Check bld gluc hourly in labor. Usually pregestational DM → IV insulin drip & titrate. Tight gluc control to avoid neonat hypoglycemia.
• Fetal lung maturity may be delayed in DM, even with reassuring FLM result.
Postpartum Management
• Usually insulin-dependent pregestational DM → resume prepregnancy regimen, or ½ of end Preg dose. GDM can stop rx, unless suspected DM 2. GDM resolves w/ deliv.
• Postpartum 75 g gluc tol test to identify nongestational DM for all GDM pts.
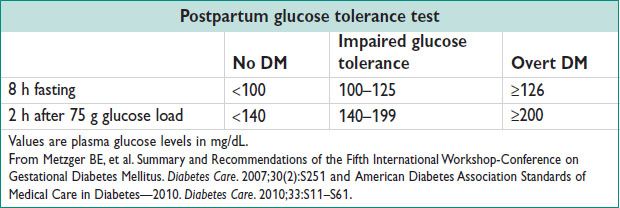
GESTATIONAL DIABETES (GDM)
Definitions, Epidemiology, and Pathophysiology
• GDM is carbohydrate intolerance w/ onset or 1st recognition during Preg
• Classification: A1GDM is diet controlled; A2GDM requires pharmacologic intervention
• GDM in ∼5–10% of pregnancies. 20–50% will → nongestational DM in 10 y; 30–50% → recurrent GDM.
• ↑ human placental lactogen/cortisol/progesterone/estrogen → ↓ periph insulin sens → impaired gluc resp → hyperglycemia. Screening per above, under Diabetes in Pregnancy.
Treatment and Medications
• See mgmt in Diabetes in Pregnancy, above.
• Oral hypoglycemics considered if dietary mgmt fails. Glyburide equiv to insulin for gluc control (starting dose: 1.25–2.5 mg twice daily → ↑ 2.5 mg as needed; max 10 mg BID). Insulin needs ↑ markedly btw 28 & 32 w gest (Obstet Gynecol 2003;102(4):857). See starting insulin schematic, above. Consider lower dose for insulin naive pt.
HYPOTHYROIDISM
Definition and Epidemiology
• Inadeq thyroid hormone to meet the requirements of periph tissues.
• Primary hypothyroidism: Hashimoto’s thyroiditis, surgical removal, radioactive ablation, invasive fibrous thyroiditis, iodine deficiency. Secondary hypothyroidism: Pituitary/hypothalamic neoplasm, trauma, ischemic necrosis (Sheehan’s syn), infxn.
• Subclinical hypothyroidism: Chronic autoimmune thyroiditis, partial thyroidectomy, radioactive iodine therapy for rx of hyperthyroidism, infiltrative disorders, drugs impairing thyroid fxn, inadeq replacement therapy for overt hypothyroidism, iodine deficiency
• 3.7% of the US pop. Females > males.
Etiology
• In women, most common cause (95%) is autoimmune (Hashimoto’s thyroiditis).
• Hashimoto’s thyroiditis: Lymphocytic thyroid infiltration → gland atrophy & fibrosis
• Subclinical hypothyroidism: Elevated TSH w/o overt hypothyroidism or low T3/T4. Early, mild thyroid failure. ∼60–80% have ⊕ antithyroid peroxidase or antithyroglobulin Abs. Progression to overt hypothyroidism in women is about 4%/y. No need to treat TSH <10 mU/L & asx.
Clinical Manifestations and Diagnosis
• Weakness, dry skin, cold intolerance, hair loss, constip, weight gain, poor appetite, dyspnea, hoarse voice, menorrhagia, paresthesias, impaired hearing
• ↑ TSH, ↓ free T4, ⊕ anti-TPO & other thyroid Abs. May also see HoNa, hypercholesterolemia, anemia, & elevated serum Cr kinase.
Treatment
• Daily levothyroxine 2 μg/kg body weight (typically 100–150 μg; start at 50–100 μg depending on severity). Adjust q4w 12.5–25 μg by TSH levels. Annual TSH levels recommended for nonpregnant.
Hypothyroidism in Pregnancy (Lancet 2012;379(9821):1142)
• Similar causes as nonpregnant. Also postpartum thyroiditis (autoimmune inflammation) → thyrotoxicosis → hypothyroidism. W/i 1-y postpartum.
• Mat hypothyroidism can ↑ SAB, placental abruption, preterm deliv, preeclampsia, mat HTN, postpartum hemorrhage, low birth weight, stillbirth, & ↓ intellectual & psychomotor dev of the fetus.
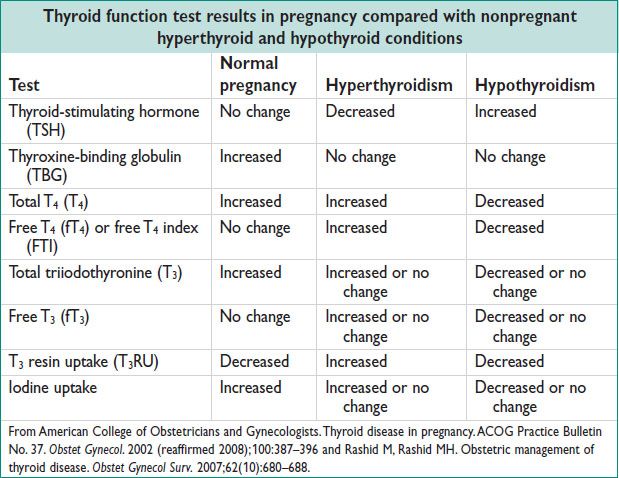
• Difficult to assess in early Preg: Total T3/T4 ↑ due to hCG cross reaction and stimulation of the TSH receptor & also ↑ TBG. In 1st trimester total T4 ↑ & TSH ↓, w/ no real hypo or hyperthyroidism.
• ACOG does not recommend routine screening of asx pregnant pts. Test if on therapy, goiter, nodularity, h/o thyroid d/o/neck irradiation, prior infant w/ thyroid dysfxn, type I DM, FHx.
• Rx similar to nonpregnant pts. Preg may ↑ thyroid hormone requirements; monit TSH & adjust.
• Treat subclinical hypothyroid → improved obstetrical outcome, but did not modify long-term neurologic dev in the fetus. Maintain TSH <2.5 mU/L in 1st trimester & <3 mU/L thereafter.
HYPERTHYROIDISM
Definition, Epidemiology, and Etiology (Endocr Pract 2011;17(3):456)
• Hyperthyroidism is caused by excess synthesis & secretion of thyroid hormone
• The prevalence of hyperthyroidism is 1.2% (0.5% overt & 0.7% subclinical)
• The most common causes are:
Graves dz (80%): Autoimmune TRAbs → bind TSH-R → ↑ TSH. Accounts for 95% of hyperthyroidism in Preg.  5–10× more than
5–10× more than  .
.
Thyroiditis (10%): Painless inflammation of thyroid due to viral infxn or postpartum inflammation → release of preformed thyroid hormone. May resolve and → hypothyroid.
Toxic adenomas: Single or multinodular, autonomously functioning, secrete thyroid hormone. More common in setting of iodine deficiency.
Other: Amiodarone, struma ovarii (ovarian dermoid), TSH secreting pituitary adenoma, gestational trophoblastic dz, follicular cell carcinoma, iodine-induced, thyrotoxicosis factitia.
Clinical Manifestation and Physical Exam (Lancet 2003;362(9382):459)
• Nervousness, anxiety, heat intolerance, tremor, palps, weight loss, oligomenorrhea, tachy, exophthalmos, thyromegaly. Thyrotoxicosis in 1 of 500 pregnancies → ↑ preeclampsia, thyroid storm, CHF, IUGR, preterm deliv, stillbirth.
• Tachy (&/or arrhythmias), HTN, warm/moist/smooth skin, lid lag, goiter, tremors
• Thyroid storm: Medical emergency, extreme hypermetabolism → seizures, arrhythmia, stupor, shock, coma. Do not delay therapy while FT4, FT3, TSH pending.
Diagnostic Workup (Endocr Pract 2011;17(3):456)
• Graves dz: ↓ TSH, ↑ FT4, ↑ FT3, ±antithyroid peroxidase Ab (TPO), ⊕TSI, ⊕TRAb, other antithyroid Abs poss.
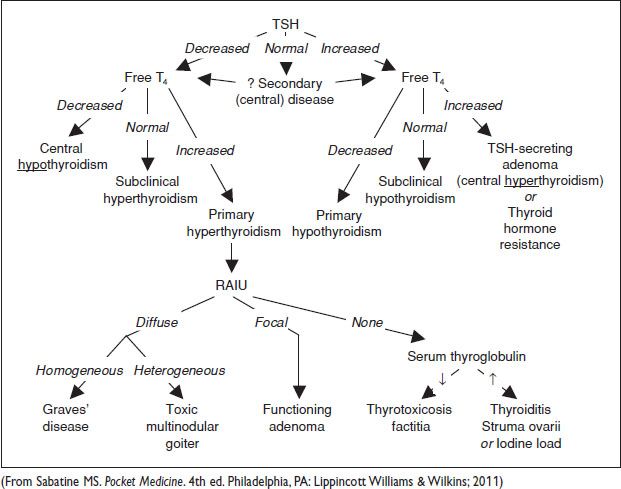
When clinical presentation is not diagnostic, RAIU is performed (J Fam Pract 2011; 60(7):388): Diffuse, homogeneous = Graves dz; diffuse, heterogeneous = toxic multinodular goiter; focal = adenoma; no uptake = thyroiditis. IgG crosses placenta → fetal Graves
• Subclinical hyperthyroidism: ↓ TSH; nml FT4 & FT3. Asx.
Treatment (Endocr Pract 2011;17(3):456)
• Symptom mgmt: b-blocker to control tachy (propranolol also blocks T4 conversion to T3)
• ATDs: PTU is 1st-line drug during the 1st trimester; blocks both iodide organification & periph T4 → T3 conversion; monit liver fxn. Methimazole okay in 2nd trimester. Titrate meds to fT4 q2–4w.
• RAI: Started for pts w/ contraindications to ATD use. Pretreat w/ methimazole prior to RAI to prevent worsening of hyperthyroidism. Contraindicated in Preg.
• Surg: For symptomatic compression, large goiter, low uptake, documented or suspected malig
Figure 17.3 Approach to thyroid disorders