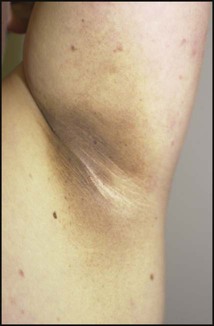
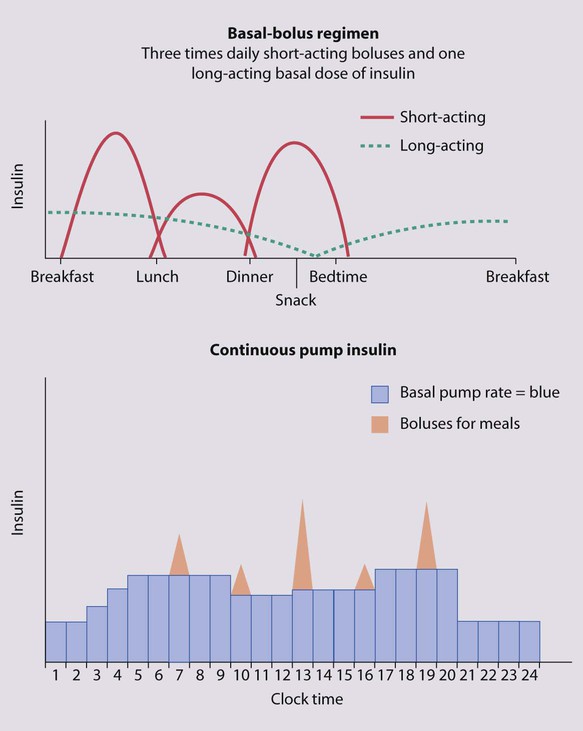
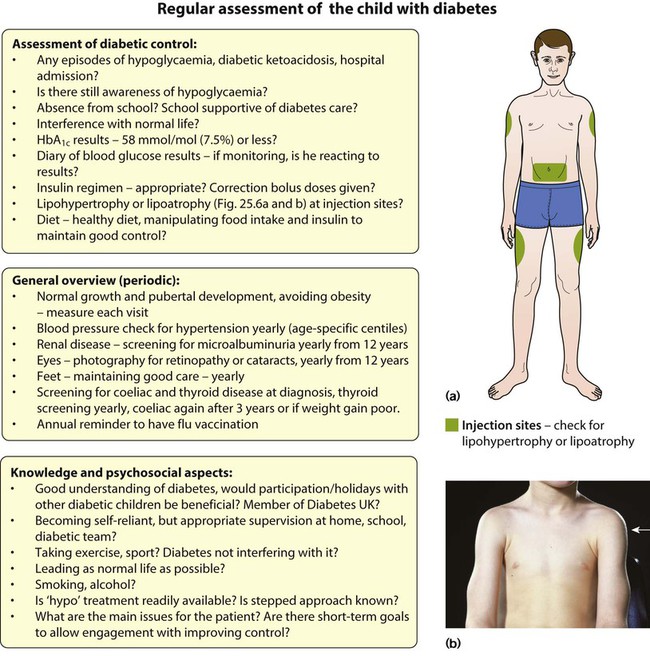
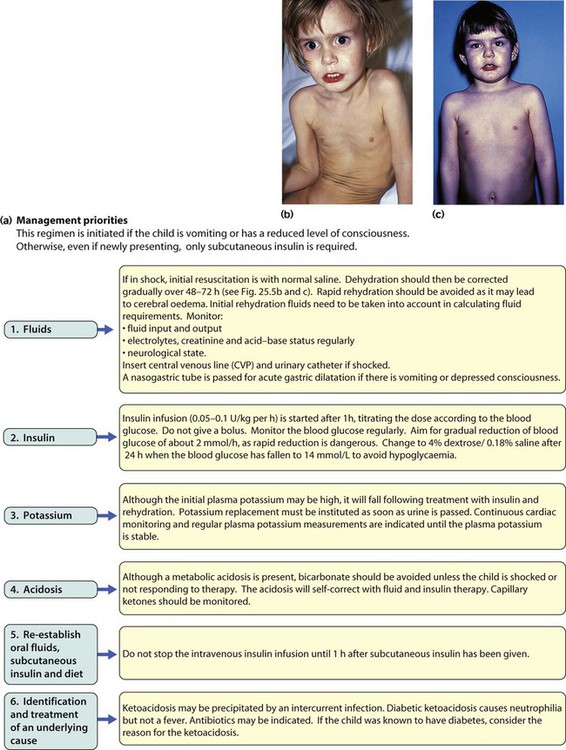
Points of note concerning endocrine and metabolic disorders in children are: • The number of children developing diabetes mellitus is increasing • The most common cause of hypothyroidism is congenital, which is detected on routine biochemical screening shortly after birth • Inborn errors of metabolism are individually very rare and managed by a few specialist centres. The incidence of diabetes in children has increased steadily over the last 20 years and now affects around 2 per 1000 children by 16 years of age. It has been estimated that the incidence of childhood diabetes will double by 2020 in developed countries. This is most likely to be a result of changes in environmental risk factors, although the exact causes remain obscure. There is considerable racial and geographical variation – the condition is more common in northern countries, with high incidences in Scotland and Finland. Almost all children have type 1 diabetes requiring insulin from the outset. Type 2 diabetes due to insulin resistance is starting to occur in childhood, as severe obesity becomes more common and in some ethnic groups. The other causes of diabetes are listed in Box 25.1. • An identical twin of a diabetic has a 30–40% chance of developing the disease • The increased risk of a child developing diabetes if a parent has insulin-dependent diabetes (1 in 20–40 if the father is affected, 1 in 40–80 if it is the mother – compared to about 1/400 in the population <16 years) • The increased risk of diabetes among those who are HLA-DR3 or HLA-DR4 and a reduced risk with DR2 and DR5. Molecular mimicry probably occurs between an environmental trigger and an antigen on the surface of β-cells of the pancreas. Triggers which may contribute are enteroviral infections, accounting for the more frequent presentation in spring and autumn, and diet, possibly cow’s milk proteins (Fig. 25.1) and overnutrition. In genetically predisposed individuals, this results in an autoimmune process which damages the pancreatic β-cells and leads to increasing insulin deficiency. Markers of β-cell destruction include islet cell antibodies and antibodies to glutamic acid decarboxylase (GAD), the islet cells and insulin. There is an association with other autoimmune disorders such as hypothyroidism, Addison disease, coeliac disease and rheumatoid arthritis in the patient or family history. There are two peaks of presentation of type 1 diabetes, preschool and teenagers. It is also commoner to present in spring and autumn months. In contrast to adults, children usually present with only a few weeks of polyuria, excessive thirst (polydipsia) and weight loss; young children may also develop secondary nocturnal enuresis. Most children are diagnosed at this early stage of the illness (Box 25.2). Advanced diabetic ketoacidosis has become an uncommon presentation (<10% in some areas of the UK), but requires urgent recognition and treatment. Diabetic ketoacidosis may be misdiagnosed if the hyperventilation is mistaken for pneumonia or the abdominal pain for appendicitis or constipation. Type 2 diabetes should be suspected if there is a family history, in children from the Indian subcontinent and in severely obese children with signs of insulin resistance (acanthosis nigricans – velvety dark skin on the neck or armpits (Fig. 25.2), skin tags or the polycystic ovary phenotype in teenage girls). As type 1 diabetes in childhood is uncommon (1–2 children per large secondary school), much of the initial and routine care is delivered by specialist teams (Box 25.3). An intensive educational programme is needed for the parents and child, which covers: • A basic understanding of the pathophysiology of diabetes • Injection of insulin: technique and sites • Diet: reduced refined carbohydrate; healthy diet with no more than 30% fat intake; ‘carbohydrate counting’, estimating the amount of carbohydrate in food to allow calculation of the insulin required for each meal or snack • Adjustments of diet and insulin for exercise • ‘Sick-day rules’ during illness to prevent ketoacidosis • Blood glucose (finger prick) monitoring and blood ketones when unwell • The recognition and staged treatment of hypoglycaemia • Where to get advice 24 hours a day • The help available from voluntary groups, e.g. local groups or ‘Diabetes UK’ • The psychological impact of a lifelong condition with potentially serious short- and long-term complications. • Human insulin analogues. Rapid-acting insulin analogues, e.g. insulin lispro, insulin glulisine or insulin aspart (trade names Humalog, Apidra and NovoRapid, respectively) – with a much faster onset and shorter duration of action than soluble regular insulin. There are also very long-acting insulin analogues, e.g. insulin detemir (Levemir) or glargine (Lantus) • ‘Short-acting’ soluble human regular insulin. Onset of action (30–60 min), peak 2–4 h, duration up to 8 h. Given 15–30 min before meals. Trade named examples are Actrapid and Humulin S • Intermediate-acting insulin. Onset 1–2 h, peak 4–12 h. Isophane insulin is insulin with protamine, e.g. Insulatard and Humulin I • Predetermined preparations of mixed short- and intermediate-acting insulins with 25% or 30% rapid-acting components. Most children are started on an insulin pump or a 3–4 times/day injection regimen (‘basal-bolus’) with short-acting insulin (e.g. Lispro, Glulisine or Insulin Aspart) being given (bolus) before each meal and snack plus long-acting insulin (e.g. Glargine or Detemir) in the late evening and/or before breakfast to provide insulin background (basal). These treatments both allow greater flexibility by relating the insulin more closely to food intake and exercise (Fig. 25.3). Patients and families are also taught how to correct any sugar above 10 mmol/L between usual meal times by extra short-acting insulin injections. However, the input required by the teams to start these intensive regimens is high, as is the need for a supportive school environment, and some patients and families still rely on twice-daily treatment with premixed insulin. The diet and insulin regimen need to be matched (Fig. 25.4). The aim is to optimise metabolic control while maintaining normal growth. A healthy diet is recommended, with a high complex carbohydrate and relatively low fat content (<30% of total calories). The diet should be high in fibre, which will provide a sustained release of glucose, rather than refined carbohydrate, which causes rapid swings in glucose levels. ‘Carbohydrate counting’ allows patients to calculate their likely insulin requirements once their food choice for a meal is known, and taking into account their pre-meal sugar level and post-meal exercise pattern. Learning this balancing act requires a lot of educational input followed by refinement in the light of experience. The aims of long-term management are: • Normal growth and development • Maintaining as normal a home and school life as possible • Good diabetic control through knowledge and good technique • Encouraging children to become self-reliant, but with adult supervision until they are able to take responsibility • The prevention of long-term complications and an HbA1c of 58 mmol/mol (7.5%) or less. These aims are difficult to achieve in all patients at all stages of their condition. Good blood glucose control is particularly difficult in the following circumstances: • Eating too many sugary foods, such as sweets taken at odd times, at parties or on the way home from school • Infrequent or unreliable blood glucose testing. ‘Perfect’ results are often invented and written down just before clinic to please the diabetes team • Illness – viral illnesses are common in the young and although it is usually stated that infections cause insulin requirements to increase, in practice the insulin dose required is variable, partly because of reduced food intake. The dose of insulin should be adjusted according to regular blood glucose monitoring. Insulin must be continued during times of illness and the urine or blood tested for ketones. If ketosis is increasing along with a rising blood sugar, the family should know how to seek immediate advice to ensure that they increase the soluble insulin dose appropriately or seek medical help for possible intravenous therapy • Exercise – vigorous or prolonged planned exercise (cross-country running, long-distance hiking, skiing) requires reduction of the insulin dose and increase in dietary intake. Late hypoglycaemia may occur during the night or even the next day, but may be avoided by taking an extra bedtime snack, including slow-acting carbohydrate such as cereal or bread. Less vigorous exercise such as sports lessons in school and spontaneous outdoor play can be managed with an extra snack or a reduction in short-acting insulin before the exercise • Eating disorders, which are common in young females with diabetes. • Family disturbance such as divorce or separation • Inadequate family motivation, support or understanding. As children can never have a ‘holiday’ from their diabetes, they need a great deal of encouragement to continuously maintain good control. Educational programmes for children and families need to be arranged regularly and matched to their current level of education. Special courses and holiday camps are available; in the UK they are organised by Diabetes UK and local groups.
Endocrine and metabolic disorders
Diabetes mellitus
Aetiology of type 1 diabetes
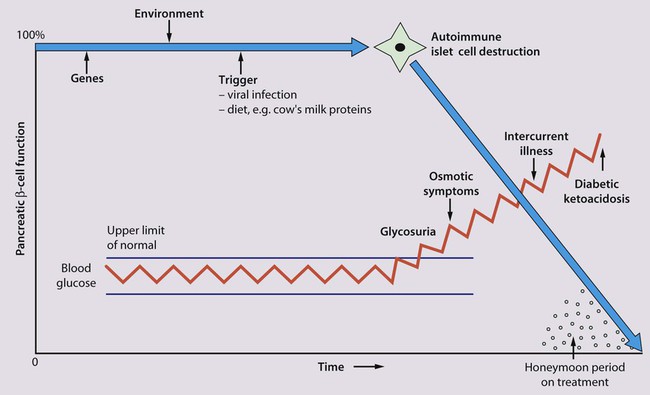
Clinical features
Diagnosis
Initial management of type 1 diabetes
Insulin
Diet
Long-term management
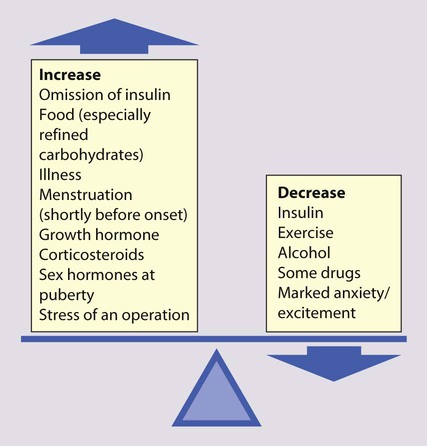
Problems in diabetic control
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine and metabolic disorders