Fig. 3.1
Progestogen versus placebo/no treatment. Outcome 3 miscarriages (women with previous recurrent miscarriage only). [Adapted from Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database Syst Rev. Has 2008;10:CD003511. With permission from John Wiley & Sons, Inc]
Even though the studies regarding progesterone supplementation in RPL are scarce and not always statistically significant, the majority of them promote the use of progesterone in women with unexplained RPL. Further research in this area is recommended and is currently being conducted.
Recommendations based on current evidence state that progesterone supplementation may be of benefit in cases of RPL, especially when the etiology is unexplained [21, 22]. In our experience the use of progesterone up until 12 weeks of gestation s hould be considered for women with RPL, and that is the common practice in our clinic.
Prolactin and Recurrent Pregnancy Loss
Measurement of prolactin levels is part of the endocrinologic evaluation of RPL.
Hyperprolactinemia affects the hypothalamic-pituitary-ovarian axis and may cause insufficient folliculogenesis and oocyte maturation and/or a short luteal phase. Increased circulating prolactin levels stimulate a generalized increase in hypothalamic dopaminergic neural activity, intended to suppress prolactin secretion but also inhibiting GnRH neurons. The end result is anovulation or an even more profound hypogonadotropic hypogonadism, depending on the extent to which gonadotropin secretion is suppressed. Mild hyperprolactinemia (20–50 ng/mL) may cause only a short luteal phase, resulting from poor preovulatory follicular development. Moderate hyperprolactinemia (50–100 ng/mL) frequently causes oligomenorrhea or amenorrhea , and higher prolactin levels (>100 ng/mL) typically result in frank hypogonadism with low estrogen levels and their clinical consequences [23].
Nevertheless, the association between hyperprolactinemia and RPL is debatable. In a case–control study, Bussen et al. evaluated the frequency of endocrine abnormalities during the follicular phase in women with a history of RPL. The concentration of prolactin in the study group of 42 women with RPL (three or more consecutive miscarriages) was significantly higher compared to the control group (42 nulligravid females with tubal or male factor infertility without miscarriage) (p = 0.015). They concluded that RPL is associated with abnormalities in prolactin secretion during the follicular phase [24].
Hirahara et al. found that treating with bromocriptine to achieve appropriate circulating levels of prolactin in women with RPL and prolactin disorder may improve subsequent pregnancy outcomes. The percentage of successful pregnancies was higher in the bromocriptine-treated group than in the group that was not treated with bromocriptine (85.7 % vs. 52.4 %, p < 0.05), and the serum prolactin levels during early pregnancy were significantly higher in patients who miscarried (31.8–55.3 ng/mL) than in patients whose pregnancies were successful [25]. On the other hand, Li et al. measured some endocrine function in the early follicular phase (days 3–5) in 144 women with unexplained recurrent (≥3) miscarriages. No association was found between recurrent miscarriage and hyperprolactinemia [26].
Although the association between hyperprolactinemia and RPL is somewhat controversial, we recommend, as do others [21], to screen for prolactin levels as part of RPL evaluation.
Polycystic Ovary Syndrome and Recurrent Pregnancy Loss
Definition and Diagnosis
Polycystic ovary syndrome (PCOS) is a complex and multifactorial disorder , first described in 1935 by Stein and Leventhal [27]. The syndrome is a combination of hyperandrogenism, ovulatory dysfunction, and polycystic ovaries. The prevalence of PCOS is 4–7 % of women of reproductive age [28–30].
In recent years several expert groups addressed the issue of defining uniform diagnostic criteria for PCOS [31], i.e., the National Institutes of Health (NIH) criteria [32], the Rotterdam criteria [33], and the Androgen Excess Society [34]. These different criteria stressed the fact that the definition for PCOS is controversial and it has a wide spectrum of clinical presentation. The most widespread diagnostic criteria are the Rotterdam criteria, which require the presence of 2 out of 3 criteria for establishing a diagnosis: (1) Oligo- or anovulation, (2) Clinical and/or biochemical signs of hyperandrogenism , (3) Polycystic ovaries (presence of 12 or more follicles in each ovary measuring 2–9 mm in diameter, and/or increased ovarian volume >10 mL) [33].
All of these diagnostic criteria require the exclusion of other etiologies (e.g., congenital adrenal hyperplasia, androgen secreting tumors, Cushing’s syndrome).
A large proportion of women with PCOS have some degree of ovulatory dysfunction, which results in oligomenorrhea or amenorrhea, and subsequent decreased infertility [35]. Hyperinsulinemia and insulin resistance are also common features among women with PCOS and are thought to have an important role in the pathophysiology of the syndrome [36]; however, it is not included in the diagnostic criteria. Moreover, in several studies that evaluated the prevalence of impaired glucose metabolism, it was found that 30–35 % of women with PCOS had impaired glucose tolerance and 7–10 % had type 2 diabetes mellitus [35, 37, 38].
Association with RPL
The prevalence of PCO morphology among women with RPL is thought to be as high as 40 % [39], although there are reports about higher prevalence [40]. Using a combination of clinical findings and ultrasound (US) or biochemical features, Yang et al. found that the prevalence of PCOS among women with RPL was even as high as 56 % [41]. On the other hand, Li et al. found that the prevalence of hyperandrogenemia in RPL was 14.6 % while ultrasound features of PCO existed in only 7.8 % [26]. This wide range is probably the result of the use of nonuniform definitions for PCOS. Cocksedge et al. examined PCOS prevalence among women with RPL, using the current recommended Rotterdam criteria for diagnosis. The study investigated a total of 300 women with RPL and found that about 10 % of women had PCOS [42].
The Mechanism for Recurrent Pregnancy Loss Among Women with Polycystic Ovary Syndrome
The exact mechanisms that may cause RPL in PCOS patients are obscure. Several etiologies have been proposed, related to the pathophysiology, endocrinology , and metabolic disturbances in PCOS. Among these are obesity, insulin resistance or hyperinsulinemia, thrombophilia-associated disorders, elevated LH, and hyperandrogenism [39, 43, 44].
Elevated BMI
Many women with PCOS suffer from obesity and various comorbidities related to it (diabetes, HTN, coronary heart disease) [35]. A body mass index greater than 30 kg/m2 increases the risk for RPL (OR: 3.5) [44, 45]. It has also been demonstrated that there is some correlation between PCOS, BMI, and RPL [46]. Weight loss among women with elevated BMI is associated with decreased pregnancy loss rates [47]. However, to date, no study has evaluated the association between weight loss and reduction in the risk for additional miscarriage in RPL patients [44].
Insulin Resistance and Hyperinsulinemia
In recent years there has been increasing interest in the role of insulin resistance and hyperinsulinemia linked to PCOS and RPL. Several studies have evaluated insulin resistance and RPL [48, 49]. In a population of women with RPL, there was found to be a higher prevalence of insulin resistance when compared with matched controls (OR: 3.55; 95 % CI 1.4–9.0) [48].
These findings were supported by studies showing that treatment with an insulin-sensitizing agent (metformin) reduced subsequent risk for miscarriage in women with RPL. Metformin lowers hepatic glucose production and increases insulin sensitivity and thereby lowers insulin blood levels. A retrospective cohort study has shown that the use of metformin during pregnancy is associated with a reduction in the miscarriage rate in women with RPL and PCOS [50]. Thereafter, a small prospective case–control clinical trial showed benefit of metformin treatment among PCOS women with RPL and abnormal glucose tolerance test [51]. Nawaz and Rizvi demonstrated, in a case–control study, that among infertile women treated with metformin there was significant decrease in the rate of pregnancy loss among women with RPL (12 % vs. 49 %; p < 0.001) [52]. However, to date, there are no randomized controlled trials assessing the role of metformin in women with RPL.
The only systematic review of randomized controlled trials concerning metformin and pregnancy loss among women with PCOS found that there was no improvement in pregnancy loss risk with metformin treatment [53].
Thrombophilic-Associated Disorders
Hyperinsulinemia and elevated activity of plasminogen activator inhibitor-1 (PAI-1) have been linked to increased incidence of miscarriage observed among women with PCOS [48, 55–57]. PAI-1 inhibits plasmin formation during plasminogen activation and subsequent fibrinolysis and has been reported to be elevated in women with PCOS. Elevated levels of PAI have been reported to be an independent risk factor for early spontaneous pregnancy loss [56]. In addition to its effects on insulin resistance, metformin lowers circulating levels of plasminogen activator inhibitor (PAI) [58].
Hyperhomocysteinemia is a common finding in women with PCOS [59] and was found to be associated with both RPL and PCOS [60]. It was shown that combined treatment with aspirin and low molecular weight heparin (LMWH) in women with hyperhomocysteinemia improved successful pregnancy rates [61]. In a small (~20 women) nonrandomized controlled study investigating women with PCOS with a history of one or more previous spontaneous miscarriages, who also had thrombophilia and/or hypofibrinolysis, it was found that the use of low molecular weight heparin along with metformin reduced pregnancy loss by 4.4-fold compared to previous gestations without treatment [62, 63].
Elevated LH and Hyperandrogenism
Hyperandrogenism and elevated LH are considered a part of the biochemical features of PCOS and have classically served a significant role in diagnosis of women with PCOS [35, 64]. In recent years, their significance in the diagnosis of PCOS has decreased, and since LH is released in pulses, abnormal levels are generally not used in order to diagnose PCOS [35].
Although elevated follicular phase LH and hyperandrogenism have been linked to RPL [39, 43], routine testing for LH and free-T in order to diagnose PCOS in patients with RPL is not recommended, since it did not predict subsequent miscarriage [65]. There was no difference in subsequent pregnancy outcome in women with prior RPL with high LH and elevated testosterone, compared to those with normal values [39, 66]. Suppressing LH secretion did not improve the outcome of pregnancy [67, 68].
PCOS is a complex entity, which encompasses a spectrum of endocrinologic and metabolic phenomena. When reviewing the literature dealing with RPL and its relation to PCOS, we may conclude that the relation of PCOS and RPL is weak; the different mechanisms suggested are just other supporting evidence for that. We can single out insulin resistance as one of the leading possible connections between PCOS and RPL, which may lead us to the notion that glucose metabolism, rather than PCOS per se, is more strongly related to the etiology of RPL.
When searching for an etiology for RPL in an index case, we should not try to reach the diagnosis of PCOS unless specific clinical features strongly suggest this entity, and aim at testing this woman’s glucose tolerance.
Treatment Options
In the committee opinion of the American Society for Reproductive Medicine (ASRM) dealing with the recommended evaluation and treatment of RPL, PCOS is mentioned to have controversial scientific evidence for its association with pregnancy loss [21].
The recommendations for PCOS women with RPL should include weight reduction when BMI is elevated, and consider metformin in a specific population of women with elevated levels of both insulin and androgen.
Weight Loss
Life style modification, specifically weight loss, has a beneficial effect on several medical conditions related to obesity and PCOS. An additional benefit of the medical recommendation for BMI reduction for obese PCOS patients might be in lowering the incidence of RPL (see Chap. 9).
Metformin Supplementation
Metformin use during pregnancy does not appear to be linked to teratogenicity or developmental disorders among exposed children studied during their first 18 months of life [62]. There is insufficient evidence to evaluate the effect of metformin in pregnancy to prevent early pregnancy loss in women with RPL. Empirical treatment may be offered only in the context of clinical trial [43].
Thyroid Disorders and Recurrent Pregnancy Loss
Thyroid disorders are among the most common endocrine disorders in women of childbearing age. Thyroid disorders are divided into (1) Hyperthyroidism—excess activity resulting in increased level of thyroid hormones (T3 triiodothyronine and T4 thyroxine) and decreased levels of TSH (thyroid-stimulating hormone), and (2) Hypothyroidism—decrease in the level of thyroid hormones with elevated TSH.
Thyroid disorders have been associated with several early pregnancy and obstetric adverse outcomes such as infertility (or subfertility), early pregnancy loss, preeclampsia, stillbirth, and preterm labor and delivery [69–71]. A certain degree of impairment in neurocognitive development has also been described in relation to overt hypothyroidism [72].
There is evidence that pregnant women express different levels of TSH and free T4, and therefore measurements of thyroid functions may require gestation-specific reference ranges, according to the pregnancy trimester [73]. This is also reinforced by the observation that in women requiring thyroid replacement therapy during pregnancy, there is an increase in levothyroxine requirement starting as early as the first trimester, and a need for close monitoring of thyroid functions throughout pregnancy [74, 75].
Hypothyroidism and Pregnancy Loss
Hypothyroidism is the second most common endocrinopathy during pregnancy, and its incidence ranges from 2 to 5 %. Autoimmune thyroiditis (also known as Hashimoto’s thyroiditis) and iatrogenic thyroid gland destruction as a therapeutic measure for hyperthyroidism are the most common etiologies for this endocrinopathy in pregnant women [74, 76, 77]. Disorders of hypothyroidism can be divided into overt and subclinical hypothyroidism, the latter usually presenting with elevated TSH and normal levels of thyroid hormones.
Observational studies have described an increased rate of first trimester pregnancy loss in women with overt and subclinical hypothyroidism. It has been shown that the risk of child loss (composite outcome for miscarriage and fetal and neonatal death) was significantly increased with the increase in TSH levels during early pregnancy, even within normal range. There was no such association between FT4 levels and the risk of child loss in the same population [78]. It has also been shown that women with subclinical hypothyroidism have a lower gestational age at miscarriage [79].
In euthyroid women, negative to thyroid autoantibodies, the rate of pregnancy loss was found to be significantly higher in women with TSH between 2.5 and 5.0 mIU/L compared to women with TSH level below 2.5 mIU/L [80]. This finding raises questions about redefining the normal range of TSH during pregnancy, especially in the first trimester, influencing risk for miscarriage.
Treatment with thyroid replacement therapy (levothyroxine ), when adequate, results in a lower miscarriage rate. In a population of women diagnosed with hypothyroidism, when levothyroxine treatment was inadequate, the outcome of pregnancy was miscarriage in 60 % of overtly hypothyroid women and in 71.4 % of subclinically hypothyroid women (p < 0.006). When treatment was adequate, term pregnancy was achieved in 100 % of overtly hypothyroid women and 90.5 % of subclinically hypothyroid women (p < 0.006) [69].
Hypothyroidism and Recurrent Pregnancy Loss
Several studies have described the relationship between hypothyroidism and RPL. The prevalence of hypothyroidism among women with a history of RPL ranges from 4 to 10 % [79, 81, 82]. The rate of subclinical hypothyroidism in the RPL population has a wider range between 7 and 29 % [82–85]. This rate is also influenced by the TSH threshold for defining subclinical hypothyroidism [80, 85].
In one observational cohort study examining over 200 women with a history of RPL, no statistically significant difference was shown with regard to the subsequent live birth rate between the subclinical hypothyroidism and euthyroid groups, nor in the treated and untreated subclinical hypothyroidism subgroups [82].
Hyperthyroidism
The prevalence of hyperthyroidism during pregnancy ranges from 0.1 to 1 %, with Graves’ disease accounting for most of the cases [74, 86]. The prevalence of hyperthyroidism among women with a history of RPL in one study was shown to be 3 % [82].
The relationship between pregnancy loss and hyperthyroidism was described mainly in reports of small numbers of subjects, and hyperthyroidism is generally not considered a major risk factor for miscarriage. Maternal hyperthyroidism before and during pregnancy was associated with a higher prevalence of spontaneous miscarriages, even when these women were treated [87]. One report of a specific familial disorder showed a higher rate of miscarriage in women affected by familial resistance to thyroid hormones (high serum concentration of free thyroxine and triiodothyronine without suppressed thyrotropin) compared to unaffected relatives [88]. Currently, there is no recommendation to routinely evaluate hyperthyroidism in women with RPL [21, 68].
Positive Anti-thyroid Peroxidase and Anti-thyroid Thyroglobulin in Women with Pregnancy Loss
In recent years, there h as been a rise in interest in the effect of thyroid autoantibodies on first trimester pregnancy loss, and more specifically recurrent pregnancy loss. It is thought that anti-thyroid antibodies exert their effect in both a TSH-dependent and a TSH-independent manner [85–89].
The prevalence of anti-thyroid antibodies in females of childbearing age is 10–18 % [83, 84, 90, 91]. In one study less than 20 % of the women with anti-thyroid antibodies were clinically hypothyroid [83]. The prevalence of anti-thyroid antibodies in women with RPL is significantly higher, between 19 and 30 % [83, 84, 91, 92].
Although in some studies the presence of thyroid autoantibodies did not affect the future risk of pregnancy loss in the population of women with RPL [92], a meta-analysis of 22 studies showed a clear association between thyroid autoimmunity and miscarriage with a pooled odds ratio of 2.5 in eight case–control studies and a pooled relative risk of 2.3 in 14 cohort studies [93]. A second meta-analysis of 31 studies published around the same time evaluated linkage between anti-thyroid antibodies and miscarriage, with 28 studies showing a positive association. When dividing the meta-analysis to cohort and case–control studies, the data in the cohort showed an odds ratio of 3.9 for miscarriage with the presence of thyroid autoantibodies. The odds ratio of miscarriage for women with RPL with positive thyroid autoantibodies was 4.22. For case–control studies the odds ratio for miscarriage was 1.8, and slightly higher in women with RPL (OR 1.86, p = 0.008) [94].
The antibodies most frequently associated with pregnancy loss and RPL are anti-thyroid-peroxidase (anti-TPO) and anti-thyroglobulin (anti-TG) [84, 91, 95]. Several studies have demonstrated that women with RPL positive for anti-thyroid antibodies also have a higher rate of other autoimmune antibodies (up to 90 %), suggesting a more general maternal immune system abnormality leading to RPL (see Fig. 3.2) [89, 91, 95].
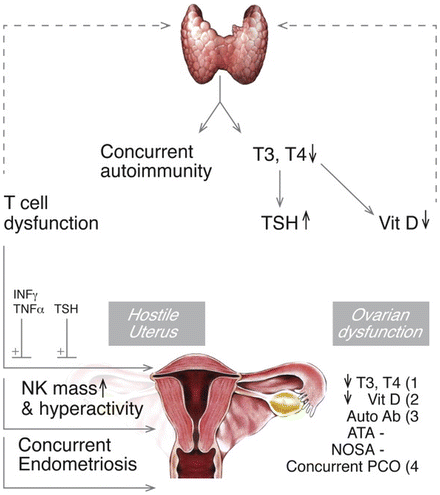

Fig. 3.2
Schematic illustration of the pathophysiological mechanisms that underlie infertility and pregnancy loss in women with hypothyroid autoimmunity. Dashed lines denote factors that potentially contribute to thyroid autoimmunity in addition to their effect on infertility (vitamin D and T cell dysfunction). For clarity, mechanisms are grouped into those that are primarily associated with hostile uterine environment and ovarian dysfunction. Concurrent autoimmunity is frequently seen in women with thyroid hypothyroidism and is associated with non-organ–specific antibodies (NOSA) in addition to autoimmune thyroid antibodies (ATA; other indirect effects are not indicated). Concurrent endometriosis and polycystic ovary are indicated due to their increased association with thyroid autoimmunity. Thyroxine, T4; Triiodothyronine, T3; Vit D, Vitamin D; TSH, Thyroid-stimulating hormone; Interferon-g, INFg; Tumor necrosis factor-a, TNF-a; Natural killer cells, NK; PCO, polycystic ovaries. [Reprinted from Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun. 2012;38(2-3):J275-81. With permission from Elsevier]
Treatment with thyroid replacement therapy in early pregnancy has been suggested in women with positive antibodies regardless of thyroid functions. In one study, in women positive for anti-TPO antibodies there was no difference in the prevalence of miscarriage between hypothyroid and euthyroid groups after treatment with l-thyroxine [84].
Screening Recommendation for Women with Recurrent Pregnancy Loss
TSH measurement, with or without thyroid hormone levels, is an inexpensive and sensitive tool for evaluation of thyroid function abnormalities. As such, it has been recommended by several clinical societies as a part of the preliminary evaluation for women with RPL [21, 22]. However, some authors recommend considering TSH measurement for the evaluation of RPL only for women with clinical signs or symptoms of thyroid abnormalities [96]. Notably, recent studies have advocated a change in the threshold for subclinical hypothyroidism, suggesting that TSH values above 2.5 mIU/L might be considered outside the normal range [21, 80].
Universal screening of thyroid functions for pregnant women is currently not recommended, since it did not result in a decrease in adverse outcomes when compared with case findings according to risk factors [97].
Recommendations for screening for thyroid autoantibodies are still inconclusive. Currently, societies dealing with reproductive medicine conclude that there is insufficient data to recommend routine screening of antibodies, especially when TSH is measured in the normal range [21, 68]. Recent clinical practice guidelines cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association state that anti-TPO measurement should be considered when evaluating patients with RPL, regardless of infertility [98].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


