Fig. 1.1
Schematic diagrams of embryonic development of the lens. (a) The optic vesicle forms in the third week of gestation and begins to gradually make contact with the surface ectoderm. (b) The lens placode forms in the fourth week of gestation. (c) The lens placode and the optic vesicle invaginate in the fifth week of gestation. (d) The lens vesicle forms completely in the sixth week of gestation. (e) The primary lens fibers form in the seventh to eighth week of gestation

Fig. 1.2
Differentiation of the lens placode cells. The peripheral cells in the lens placode terminally differentiate into the anterior subcapsular epithelium, the central cells terminally differentiate into primary lens fibers, and the cells at the junction of the anterior and posterior wall terminally differentiate into the equatorial epithelium and form the secondary lens fibers
1.1.3 Development of Lens Epithelium
Although the mature lens develops from the same lens placode cells, the structures of its various parts are different to some extent. Beneath the anterior lens capsule is a monolayer of epithelial cells, bilateral equatorial zones are constituted by spindle-shaped cells, and there are no cells beneath the posterior lens capsule. To know the reasons for this different differentiation, we need to retrace the morphogenesis of the lens placode. The lens placode cells originate from the surface ectoderm overlying the optic vesicle, and they are the primitive stem cells of the lens. The lens placode is a monolayer of primitive cells and different parts of cells vary in morphology and size. In the lens placode, the cells approximating the center, i.e., the basement cells of the vesicle pit, show a greater level of differentiation and become columnar. The more peripheral cells, which will transform into the anterior surface cells of the lens vesicle, are round and exhibit a lower level of differentiation, as well as features of stem cells. The cells on the peripheral edges are adjacent to the corneal epithelial stem cells, and a potential association exists between the development of these two types of cells.
In the sixth to seventh week of gestation, the lens epithelial cells are visible. However, morphologically, they are not the typical monolayer cells but pseudostratified cells with active proliferation. From the fourth month to birth, these epithelial cells remain mostly unchanged.
1.1.4 Formation of Lens Fibers
The lens fibers are divided into primary lens fibers and secondary lens fibers.
1.1.4.1 Primary Lens Fibers
After the lens vesicle separates from the surface ectoderm, the differentiation of the epithelial cells in the vesicle accelerates. The cells of the posterior wall expand and tend to be fusiform shaped. They protrude from the posterior wall toward the center of the lens vesicle, with their nuclei gradually migrating from the center to the front of the cells. Then, the cells gradually elongate and their nuclei move close to the cellular equator. During this process, the lumens in the lens vesicle are getting narrower and narrower, changing gradually from an empty sphere to an arc-filled or crescent-filled sphere. As the fusiform lens fiber cells reach the anterior subcapsular epithelium, the vesicle lumen disappears and a solid sphere comes into being. The nuclei in the elongated fiber cells gradually disappear and finally, the cells differentiate completely into fibers, which are referred to as the primary lens fibers. Thus, the primary lens fibers become the embryonic nucleus. Embryonic nuclear cataract is caused by the malformation of the front apices of the primary lens fibers in the sixth to the eighth week of gestation and manifests as small, sporadic white opacified dots in the central lens. It is less likely to affect visual acuity.
1.1.4.2 Secondary Lens Fibers
In the seventh week of gestation, the epithelial cells derived from the lens equatorial zone begin to differentiate, become spindle shaped, and migrate toward the central core of the lens vesicle. Their anterior pole grows toward the anterior subcapsular epithelium and their posterior pole toward the posterior capsule, meeting the lens fibers coming from the opposite direction at the posterior and anterior poles of the lens. These fibers lie tightly outside the primary lens fibers and encircle the latter layer by layer. The secondary lens fibers encircling the embryonic nucleus are also known as the fetal nucleus. If the lens is impaired in the third month of gestation, fetal nuclear cataract will occur and manifest as opacity between the anterior and posterior Y sutures, which is often combined with embryonic nuclear cataract and impacts visual acuity significantly.
1.1.5 Formation of the Lens Capsule
The lens capsule is a basement membrane formed by the accumulated lamina of substances secreted by lens epithelial cells, and its components mainly include laminin, fibronectin, collagen type IV, and sulfated glycosaminoglycans [2]. In the fifth week of gestation, the homogeneous, transparent, integrated, and ultrathin capsular membrane begins to form. In the seventh week, the structure of the lens capsule is clearly visible. In the tenth week, the thickness of the anterior and posterior polar region is almost the same. In the following period, the thickness of all parts of the capsule will also increase with lens development.
1.1.6 Formation of the Lens Sutures
In the eighth week of gestation, the formation of the lens sutures begins. The lens sutures are the Y-shaped structures, derived from the equatorial secondary lens fibers ending at the specific locations of the anterior and posterior poles (Fig. 1.3). The fibers, which proceed to the fork of the Y suture at the anterior pole, extend to the apex of the Y suture at the posterior pole and vice versa. The lens fibers become tapered and flared at the ends and connect with contralateral fibers precisely. After the Y sutures are formed, the lens gradually becomes ellipsoidal. From the third trimester to after birth, the lens sutures become irregular and appear as complex branches with the growth of the lens and the elongation of lens fibers. Lens impairment in the third month of gestation may lead to sutural cataract, manifesting as opacification of the anterior and posterior sutures.
After the formation of the embryonic nucleus, the new fibers derived from lens epithelial cells at the equatorial zone encircle the previously formed lens sutures, forming a regular and layered structure. If the lens is impaired after the formation of the fetal nucleus, lamellar cataract may occur, manifesting as a white circular opacity surrounding the fetal nucleus. It is shaped like a white shell, which is concentric with the lens capsule. It is transparent within the shell, as well as the outer lens cortex. The arrangements of lens fibers at different developmental stages determine the layered appearance of the lens. The layers of an adult lens, which can be distinguished in a slit-lamp biomicroscopic section, include embryonic nucleus, fetal nucleus, adult nucleus, and cortex (Fig. 1.3).
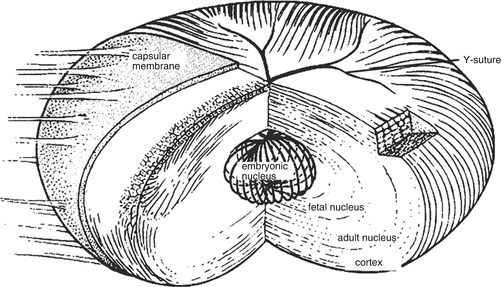

Fig. 1.3
Lens sutures and the layered lenticular structure. Y sutures are formed by lens fibers at the anterior and posterior poles of the lens. The layers of the lens from the core to the surface are embryonic nucleus, fetal nucleus, adult nucleus, cortex, and lens capsule
1.1.7 Formation of the Vascular Sheath of the Lens
Around the embryonic lens, there is a complex network of vessels, which provides nutrition for the embryonic development of the lens and is referred to as the vascular sheath of the lens. In the first month of gestation, the hyaloid artery branches into many confluent vessels, which form a vessel network that covers the entire posterior surface of the lens and is known as the posterior vascular sheath of lens. The capillaries, developing from the branches of the posterior vascular sheath, grow to the equatorial zone of the lens and anastomose with the choroidal veins, forming the capsulopupillary zone of the vascular sheath. Braches from this zone anastomose with the long posterior ciliary artery and form the anterior vascular sheath of the lens. This anastomosis of vessels is also referred to as the pupillary membrane. These vessel networks are completely developed in the ninth week of gestation and will gradually regress with fetal development and disappear by birth. If the posterior vascular sheath fails to regress completely, it will manifest as a small patch of opacity on the posterior capsule, which is known as a Mittendorf dot. Clinically, the remnant of the anterior vascular sheath of lens can also be seen, manifesting as a remnant of linear pigmented tissue in the pupillary zone.
The lens continues to grow and develop after birth and it grows most rapidly in the first year [3–5]. Then the growth gradually slows down from 1 to 10 years old and continues after the age of 10 but in an extremely slow manner. The lens becomes approximately 0.5 mm thinner before the age of 10 and this usually happens before the age of 3. The radii of anterior and posterior surface increase by 1.0 mm and 0.2 mm, respectively. These changes may be caused by the lens being passively stretched by its equatorial growth, which flatten the lens surface curvature and eventually leads to the decrease of refractive power [6].
1.2 The Main Regulatory Factors of Lens Development
Lens development is regulated by multiple transcription factors and signaling pathways. Abnormal expression of transcription factors and the aberration of signaling pathways may lead to lens dysplasia and cataract. It is essential to study the spatiotemporal regulatory network of the lens development, which in turn will facilitate to better understand the molecular mechanism of lens disorders. Currently, several important transcription factors have been found to be involved in lens development.
1.2.1 PAX6
The PAX6 gene is a highly conserved paired box gene, which acts as a “master control” regulator for ocular development. The products of PAX6 are DNA-binding proteins and transcription factors. PAX6 is expressed as early as in the precursor cells of the lens placode and is essential for lens placode formation [7]. PAX6 proteins are classified within a sparse group of “master” regulatory proteins, including BSAP/Pax5, Gata1, Gata2, MyoD, PU.1, Runx2, Sox9, and a few others, that work at the top of genetic networks as “molecular switches” that control cell type specification and differentiation. The PAX6 function is dosage dependent, such as haploinsufficiency. Mutation or loss of one allele in humans leads to a spectrum of eye abnormalities including lack of iris, cataract, corneal opacification, and neovascularization, as well as optic nerve hypoplasia [8]. The first notable phenotype of PAX6 deficiency was reduced size of the eye with progressively deteriorating eye morphology in mouse or rat. PAX6 regulates the expressions of various transcription factors, such as Sox2, Maf, and Prox1, which, in turn, regulates lens fiber differentiation and lens formation [9]. During the lens fiber differentiation, PAX6 ensures the differentiating cells exit from cell cycle, elongation, and expression of lens fiber-specific proteins, such as crystallins, and complete the lens formation. PAX6 can also act cooperatively with transcription factors, like Maf, Prox1, Sox2, and Six3, and exert its function via other factors like pRB and Mitf. Xia and colleagues examined the expression of PAX6 at different developmental stages of the lens in mice and found that PAX6 was expressed on embryonic day (E12.5) and E17.5 and on days 10, 20, and 60 after birth [10]. The expression of PAX6 was evenly expressed in lens epithelium. The results suggest that PAX6 is not only required for lens embryonic development but also essential for the continuing lens fiber differentiation after birth. In vitro studies also confirmed that the normal expression of the PAX6 gene was vital for the proliferation and differentiation of lens epithelial cells [11]. Furthermore, PAX6 is essential for lens fiber regeneration after cataract surgery [12].
1.2.2 Maf
The Maf proteins are a family of transcription factors containing the basic region of bZIP (basic region-leucine zipper). Two copies of the Maf protein or a copy of Maf protein and a copy of another related protein form a dimer, which can bind to specific DNA sequences. Members of the Maf family include L–Mafs, C–maf, V–maf, MafB, and NRL. In 1998, it was discovered for the first time that L–Maf, a member of the Maf gene family, plays a key role in lens development [5]. It affects the chick αA-crystallin expression by regulating the enhancer αCE2 [13]. Later studies discovered that C–maf and MafB of the Maf family also participate in lens development [14–16]. In addition, studies also confirmed that the missense mutation of the Maf gene can cause congenital cataract [17]. Hence, the Maf gene family directly participates in lens development and their mutations may cause cataract. However, recent studies have also shown that for lens development, only C–Maf is essential, while L–Maf and MafB appear redundant [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


