Observation
References
Number of patients and mode of clinical characterisation
Design of the study
Individual main findings
The prevalence increases from at-risk mental states to first-episode samples to chronic course of schizophrenia
At-risk mental state samples
Summary of previous literature (Zink et al. 2014a)
Review about 11 individual studies including 1,590 patients (regarding OCD) or 553 patients (regarding OCS)
Cross-sectional assessment of OCD or OCS using different diagnostic procedures
Weighted for sample sizes of individual reports, ARMS patients fulfil criteria for OCD in only 5.2 % and for OCS in 13.7 %
233 ARMS patients presenting BS, APS and BLIPS
Cross-sectional assessment of OCS according to SCID I
5.2 % of PREVENT patients, the patients fulfil criteria of OCD and 11.2 % for OCS
First episode patient
Review about 6 individual studies including 630 FEP patients (ad OCD) or 578 FEP patients (ad OCS)
Cross-sectional assessment of OCD or OCS using different diagnostic procedures
Weighted for sample sizes of individual reports, 7.3 % of FEP patients fulfil criteria for OCD and 17.1 for OCS
Strakowski et al. (1993)
27 FEP
Cross-sectional assessment of comorbid conditions at first hospitalisation using SCID
7.4 % of FEP fulfil criteria of OCD Tendency towards longer duration of hospitalisation
Strakowski et al. (1995)
18 FEP
Cross-sectional assessment of comorbid conditions at first hospitalisation using SCID
11 % of FEP fulfil criteria of OCD
Craig et al. (2002)
225 FEP
Cross-sectional assessment of comorbid conditions at first hospitalisation using SCID
4 % of FEP fulfil criteria of OCD and 21 % of OCS
Schizophrenic patients
Mukhopadhaya et al. (2009)
Review of previous studies including 1972 patients
Cross-sectional assessments of OCS based on SCID, YBOCS, HZI, PADUA and other scales
High variability and mean prevalence of OCS in 22 % of schizophrenia patients
Buckley et al. (2009)
Review covering 3656 patients
Cross-sectional assessment of OCS in schizophrenia
Mean prevalence rate of 23 %
Lysaker and Whitney (2009)
Not specified
Review of studies reporting on OCS prevalence in schizophrenia
Amongst schizophrenic patients, more than one-third suffers from clinically significant OCS, 10–25 % meet diagnostic criteria of OCD
Swets et al. (2013)
Review of 43 individual studies about 3978 patients
Review of studies reporting OCS and OCD prevalence rates in schizophrenia
Mean prevalence for OCD: 12.3 % (13.6 % after adjustment in meta-regression), for OCS 30.7 % (30.3 % adjusted in meta-regression). Higher prevalence rates in studies using DSM IV, YBOCS assessment and including patients with longer duration of illness
Observation of de novo onset or exacerbation of OCS during antipsychotic treatment
9 SCH patients
Longitudinal observation of course of illness
First manifestation and start of antipsychotic treatment precede onset of OCS
De Haan et al. (1999)
121 recent-onset SCH patients
Longitudinal observation of course of illness
Emergence or increase of OCS in 1.3 % of non-clozapine-treated and 20.6 % of clozapine-treated patients
Lykouras et al. (2003)
55 SCH patients
Systematic review of published case reports
Until 2003, a de novo onset or exacerbation of OCS had been published regarding clozapine (N = 30), risperidone (N = 16), olanzapine (N = 8) and quetiapine (N = 1)
De Haan et al. (2004)
200 recent-onset SCH patients
Longitudinal observation of course of illness
Emergence or increase of OCS in 0 % of non-clozapine-treated and 9.8 % of clozapine-treated patients
Proportion of SGA-induced OCS within the complete comorbid sample
Lin et al. (2006)
CLZ: 102
Cross-sectional. Stratification for CLZ treatment with or without OCS
Within 39 clozapine-treated patients with OCS, 29 were classified as clozapine-induced
Lim et al. (2007)
Total sample: 209, comorbid subsample: 26
Cross-sectional. Stratification for SZ with or without OCS
Within 26 SGA-associated schizophrenics with OCS, only 3 had a history of transient OCS before the onset of psychosis
Schirmbeck et al. (2011)
CLZ: 26
Cross-sectional. Stratification for treatment with SGAs in monotherapy
Within 39 patients, 28 showed OCS, but only 3 reported OCS before or at onset of psychosis
OLZ: 13
Assumption of different clinical features
Doyle et al. (2014)
CLZ: 62
Qualitative evaluation of OCS using the OCI
Two of fourteen OCS-positive, CLZ-treated patients suffered from OCS before start of CLZ treatment (14 %). OCS prevalence in CLZ group: 22 %. No correlation of CLZ dosage with OCS severity. OCS-positive CLZ-treated patients present pronounced doubting, OCD patients pronounced washing
OCD: 35
Kim et al. (2012)
51 schizophrenia patients with SGA-induced OCS
Comparison of symptom structure to primary OCD patients using the YBOCS
Similar to primary OCD patients, five identical factors (forbidden thoughts, hoarding, cleaning, symmetry, and counting) accounted for 70.7 % of the total variance
Michalopoulou et al. (2014)
Schizophrenia patients with (N = 20) or without (N = 20) OCS and 20 matched controls
Comprehensive neurocognitive characterisation
Lower performance in processing speed discriminated patients with or without OCS, but did not correlate with OCS severity. No differences in other neurocognitive domains
Table 10.2
Evidence for SGA-induced OCS derived from pharmacological investigations
Argument | Reference | Number of patients | Design | Main findings |
|---|---|---|---|---|
Association of CLZ with comorbid OCS | Lim et al. (2007) | Total sample: n = 209, comorbid subsample: n = 26 | Cross-sectional. Stratification for SZ with or without OCS | CLZ treatment in 35.9 % of the total sample, but in 76.9 % of the comorbid patients |
Association of OCS with OLZ or CLZ | Sa et al. (2009) | CLZ: n = 40 | Cross-sectional. Stratification for treatment with CLZ or HAL | Prevalence of OCS 20 % (CLZ) vs 10 % (HAL). Higher severity of OCS with CLZ |
HAL: n = 20 | ||||
Ertugrul et al. (2005) | CLZ: n = 50 | Cross-sectional. Stratification of treatment with CLZ | Within 50 patients treated with CLZ, 76 % showed OCS. 20 % reported retrospectively de novo onset and 18 % an exacerbation | |
Schirmbeck et al. (2011) | CLZ: n = 26 | Cross-sectional. Stratification for treatment with SGAs in monotherapy | Prevalence of OCS 71.8 % in CLZ or OLZ vs 9.7 % in AMS or APZ. Highest severity of OCS with CLZ | |
OLZ: n = 13 | ||||
AMS: n = 15 | ||||
APZ:n = 16 | ||||
Scheltema Beduin et al. (2012) | CLZ: n = 28 | Cross-sectional. Stratification for treatment with SGAs in monotherapy | Significant differences in OCS prevalence between groups stratified for mode of treatment CLZ (prevalence of OCS: 38.9 %), OLZ (20.1 %), RISP (23.2 %) and untreated patients ( 19.6 %) | |
OLZ: n = 41 | ||||
RISP: n = 36 | ||||
Untreated: n = 22 | ||||
Correlation of OCS with duration of treatment | Lin et al. (2006) | CLZ: n = 102 | Cross-sectional. Stratification for CLZ treatment with or without OCS | Duration of CLZ treatment significantly longer in CLZ-OCS patients (82 vs 56 months), no difference in duration of illness |
Schirmbeck et al. (2011) | CLZ: n = 26 | Cross-sectional. Stratification for CLZ monotherapy | Duration of CLZ treatment correlates positively with OCS severity (YBOCS, R = 0.59) | |
Scheltema Beduin et al. (2012) | CLZ: n = 72 | Cross-sectional. Stratification for CLZ treatment with or without OCS | Patients treated with CLZ for longer than 6 months suffer from OCS more often than patients recently switched to CLZ (47.3 % vv 11.8 %) | |
Correlation of OCS with CLZ dosage or plasma concentration | Reznik et al. (2004) | n = 15 | Cross-sectional. Stratification for CLZ therapy | Dosage-related, pro-obsessive influence of CLZ |
Mukhopadhaya et al. (2009) | n = 59 | Cross-sectional. Stratification for CLZ therapy | Higher CLZ dosage in patients with comorbid OCS (432 mg/day) than without (351 mg/day) | |
Schirmbeck et al. (2011) | CLZ: n = 26 | Cross-sectional. Stratification for CLZ monotherapy | CLZ dosage correlates positively with OCS severity (YBOCS, R = 0.50) | |
Lin et al. (2006) | CLZ: n = 102 | Cross-sectional. Stratification for CLZ treatment with or without OCS | Higher plasma concentrations in CLZ-treated patients with OCS (595 ng/L) than without OCS (434 ng/L) | |
Improvement after CLZ dose-reduction in combination with APZ or ZIPR | Rocha and Hara (2006) | n = 3 | Longitudinal observation of OCS severity | Reduction of OCS severity after CLZ down-tapering in combination with APZ |
Zink et al. (2006) | n = 1 | Longitudinal observation of OCS severity | Reduction of OCS severity from YBOCS 24 to 19 after reduction of CLZ from 500 to 250 mg/die and combination with APZ (30 mg) | |
Englisch et al. (2009) | n = 7 | Longitudinal observation of OCS severity | Reduction of OCS severity from YBOCS 19 to 12 after reduction of CLZ from 364 to 293 mg/die and combination with APZ (23 mg) | |
Villari et al. (2011) | n = 2 | Longitudinal assessment of OCS severity | Improvement of OCS severity in two cases after add-on of APZ | |
Eryilmaz et al. (2013) | n = 4 | Longitudinal assessment of OCS severity | Improvement of OCS severity in four cases after add-on of APZ | |
Krause et al. (2013) | n = 6 | Longitudinal assessment of OCS severity | Improvement of OCS severity in six cases after add-on of ZIPR | |
Increase of OCS severity during treatment with CLZ or OLZ | Schirmbeck et al. (2013) | n = 75 | Longitudinal observation of OCS severity | CLZ progressively aggravates OCS. A significant time effect discriminates between groups treated with CLZ/OLZ or AMS/APZ |
Modulation of brain activation by SGA treatment | Schirmbeck et al. (2014) | n = 40 | Comparison of brain activation in fMRI | Patients treated with CLZ or OLZ did not differ in brain activation patterns during a working memory task from samples treated with AMS or APZ but showed significantly increased activation in the orbitofrontal cortex (OFC) during response inhibition |
Improvement of OCS severity by serotonergic treatment | Poyurovsky et al. (1999) | Interventional clinical trials | Assessment of influences of serotonergic antidepressants on OCS severity | Improvement of obsessions, positive and negative psychotic symptoms by fluvoxamine |
Stryjer et al. (2012) | 15 schizophrenia patients with comorbid OCD | Interventional clinical trial with escitalopram 20 mg/die for 12 weeks | Significant improvement in YBOCS total score, obsessions and compulsion, improvement in PANSS positive and negative scores, particularly regarding anxiety, tension, depression and preoccupation |
10.2.2.1 Epidemiological Evidence
High Prevalence Rates of OCS in Schizophrenia After Market Approval of SGAs
The co-occurrence of OCS did not gain much clinical awareness in scientific publications as long as treatment with different FGAs was the first-line-therapy in schizophrenia. Only few studies reported observed comorbidities during FGA treatment (Berman et al. 1995a, b; Fenton and McGlashan 1986; Nolfe et al. 2010). With the approval of SGAs, the situation markedly changed. CLZ was introduced for the treatment of schizophrenia in the 1970s in Europe and the late 1980s in the USA (Hippius 1989; Kang and Simpson 2010). After this paradigmatic change in treatment, the prevalence estimations markedly increased. A recent meta-analysis concludes that 13.6 % of patients with schizophrenia fulfil the criteria for comorbid OCD and 30.3 % experience at least mild OCS severity (Swets et al. 2013) (see Fig. 10.1 and Chap. 4). Even if a potential publication bias and an increased general awareness have to be considered, the high numbers may indirectly reflect an interrelation between CLZ treatment and OCS.
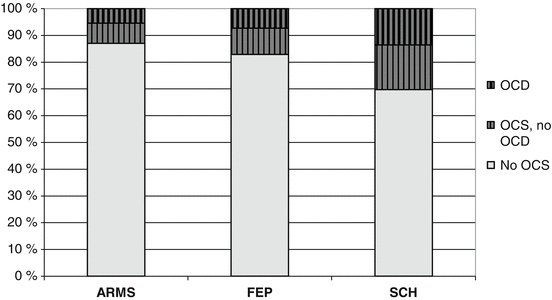

Fig. 10.1
Data on prevalence of OCS and OCD in different stages of psychotic disorders strongly suggest that during progress of illness and its treatment, pro-obsessive factors are active. One of them might be the antipsychotic pharmacotherapy. Data were extracted from epidemiological publications as summarised in (Zink et al. 2014a), the PREVENT trial and the meta-analysis of Swets et al. (2013). From left to right: Prevalence rates in at-risk-mental state studies (ARMS). Prevalence rates in first-episode psychotic patients. Prevalence rates in schizophrenia patients
Higher OCS Prevalence Rates During Later Stages of the Psychotic Illness
As described in more detail within Chap. 4, prevalence rates of OCS/OCD increase during the course of psychotic illness. During the ARMS only 5.42 % (CI: 4.41–6.43) of investigated patients fulfilled the criteria for OCD and only 12.98 % (CI: 10.63–15.33) reported OCS (Zink et al. 2014a). These rates are considerably lower compared to 7.3 % OCD and 17.1 % OCS prevalence rates in first-episode patients and even lower to the above mentioned rates of 13.6 and 30.3 % in later disease stages (Swets et al. 2013) (see Fig. 10.1). As the disease progresses patients are more likely treated with CLZ and are cumulatively exposed to higher dosage and longer treatment duration. The observed increase in prevalence and severity of OCS over the course of psychotic illness could partly be attributed to the proposed pro-obsessive effects of CLZ.
Observation of De Novo OCS or Marked Aggravation During Antipsychotic Treatment
A growing number of case reports and case series report the mentioned sequential order of the events, “onset of psychosis”, “start of antipsychotic treatment” and “onset or aggravation of OCS”, as recently summarised (Schirmbeck and Zink 2012). Most studies describe this pattern during treatment with CLZ; however, the de novo occurrence of OCS has also been reported during other SGAs such as OLZ and risperidone (de Haan et al. 2002; Kulkarni et al. 2012; Lykouras et al. 2003; Poyurovsky et al. 2004). Unfortunately, prospective investigations with randomised assignment to different modes of SGA treatment are still missing, and studies are limited by retrospective assessments (Schirmbeck et al. 2011) or narrative design (Lykouras et al. 2003). Prospective observational studies confirm an increase in OCS severity in patients treated with CLZ or OLZ (de Haan et al. 2002; Schirmbeck et al. 2013) (see Sect. 10.2.2.2).
Estimated Proportions of SGA-Induced OCS Within Comorbid Samples
Several authors have investigated the proportion of SGA-associated OCS within their comorbid samples. In their review Poyurovski et al. concluded that 70 % of investigated cases experienced de novo emergence of OCS during treatment with pro-obsessive SGAs (Poyurovsky et al. 2004). Accordingly, Lykouras et al. reviewed published data and reported de novo OCS in 77 % of CLZ-treated patients (Lykouras et al. 2003). Other investigations resulted in even higher numbers of second-onset OCS within investigated samples: 29 of 39 (74 %) (Lin et al. 2006), 23 of 26 (88 %) (Lim et al. 2007), 25 of 28 patients (89 %) (Schirmbeck et al. 2011) and 12 of 14 (86 %) (Doyle et al. 2014). These numbers further argue in favour of a potential causal interrelation between SGAs, especially CLZ and OCS occurrence.
10.2.2.2 Pharmacological Evidence
Apart from these epidemiological data, additional pharmacological arguments support the assumption of SGA-induced OCS (see also Table 10.2).
The Differential Effect of Pharmacodynamic Properties on OCS Comorbidity Rates
When comparing schizophrenia patients who have been grouped according to pharmacodynamics properties of their antipsychotic treatment, the risk for comorbid OCS markedly differs. High prevalence rates in CLZ-treated patients contrast with low rates during treatment with FGAs, for instance, haloperidol or other SGAs (Ertugrul et al. 2005; Sa et al. 2009; Scheltema Beduin et al. 2012).
SGAs differ in their specific pharmacodynamic properties, in particular, regarding inherent serotonergic and dopaminergic blockade, monoaminergic reuptake inhibition or even partial serotonergic agonism (Lopez-Gil et al. 2010; Meltzer and Sumiyoshi 2008; Remington 2008; Shapiro et al. 2003). CLZ and OLZ can be described as potent serotonergic antagonists combined with rather weak antidopaminergic effects (Bymaster et al. 1997; Meltzer et al. 2003). Substances such as amisulpride (AMS), a dopamine D3/D2 receptor antagonist (Kim et al. 2008; Pani et al. 2008) with inherent predominantly dopaminergic blocking activities (Scatton et al. 1997) and the partial dopaminergic and serotonergic agonist aripiprazole (APZ) (Sparshatt et al. 2010) have even been proposed to have a potential anti-obsessive effect in patients with schizophrenia (Chang et al. 2008; Englisch et al. 2009; Englisch and Zink 2008; Zink et al. 2006).
Consequently, comparing OCS frequency and severity in groups treated with CLZ/OLZ vs AMS/APZ is of particular interest to elucidate pharmacodynamic effects. A recent study found that more than 70 % of patients within the first group reported OCS compared to less than 10 % of patients treated with AMS or APZ (Schirmbeck et al. 2011). Vice versa, if schizophrenia patients were grouped according to the presence or absence of comorbid OCS, 77 % of comorbid individuals, but only 36 % of non-comorbid patients were treated with CLZ (Lim et al. 2007). However, the cross-sectional design of these studies precludes causal conclusions and confounding effects due to the selection of specific SGAs for specific subgroups of patients have to be considered.
Longitudinal Effects of SGAs on OCS Severity
Schirmbeck et al. tried to overcome these limitations by investigating potential differential effects of the abovementioned pharmacodynamically diverse SGA groups over time. Integrating the two factors “group” and “time” into their statistical analysis resulted in a significant interaction effect (completer analysis: p = 0.006; full sample analysis: p = 0.007). Whereas the CLZ/OLZ group showed persistently high OCS severity over a 12-month observational period, the AMS/APZ group reported a significant decrease in symptom severity (Schirmbeck et al. 2013).
Treatment Duration and Dose-Related Variation in OCS
Several studies added to the current state of research by investigating associations between OCS severity and duration, as well as dosage and serum levels of CLZ treatment. Findings suggested dosage-related effects.
Lin et al. (2006) compared CLZ-treated patients with vs without comorbid OCS and found significantly longer treatment durations in the comorbid group. Noteworthy, groups did not differ regarding duration of illness. In accordance, Schirmbeck et al. reported a positive correlation between OCS severity and duration of CLZ treatment (Schirmbeck et al. 2011), and Scheltema et al. found increased OCS prevalence rates in patients treated with CLZ for longer than 6 months (Scheltema Beduin et al. 2012). Similar observations were reported by de Haan et al. regarding the closely related SGA OLZ. Again severity of OCS was significantly associated with longer duration of treatment (de Haan et al. 2002).
Several authors further demonstrated that higher levels of OCS severity were correlated with higher dosage and/or blood serum level of CLZ (Lin et al. 2006; Mukhopadhaya et al. 2009; Reznik et al. 2004; Schirmbeck et al. 2011). In addition, the reduction of daily CLZ dosage, for instance, using a combination with APZ or ziprasidone, was accompanied by an alleviation of OCS severity (Englisch et al. 2009; Eryilmaz et al. 2013; Krause et al. 2013; Rocha and Hara 2006; Villari et al. 2011; Zink et al. 2006). This observed improvement in OCS may represent both a reduction of dose-related side effect of CLZ and the proposed favourable effect of APZ. Anti-obsessive effects of APZ were suggested in a placebo-controlled randomised trial, which revealed reduced OCS severity after combination with APZ during constant CLZ dosage (Chang et al. 2008).
It has to be acknowledged that contradicting findings have been published: An alleviation of OCS severity has been observed after addition of CLZ (Peters and de 2009), an increase in CLZ dosage (Lykouras et al. 2003) or start with OLZ treatment (Poyurovsky 2013; van Nimwegen et al. 2008). These controversial findings might be explained by differences in sample characteristics.
Due to mentioned diagnostic difficulties (Chap. 3), psychotic symptoms might have been misperceived as OCS. Patients with treatment-resistant schizophrenia or patients who exhibit obsessive ruminations, stereotypic thoughts or repetitive ritualised behaviour clearly related to the content of delusions or hallucinations might indeed benefit from treatment with OLZ or CLZ.
Beneficial effects of SGAs have also been reported in primary OCD, especially in cases with treatment resistance to serotonergic antidepressants (Bandelow et al. 2008; Bloch et al. 2006; Dold et al. 2011; Muscatello et al. 2011). However, CLZ has not been found effective in OCD (McDougle et al. 1995), and no treatment guidelines recommend CLZ as an augmentation strategy in this group of patients (Kordon et al. 2011).
In summary, cumulative evidence and especially the dosage-dependent drug-OCS interaction strongly suggest a causal interrelation. The vast majority of investigations involved CLZ, suggesting that OCS can be considered an AE of SGAs with strong anti-serotonergic mechanisms. To further validate the concept of SGA-induced OCS, recent investigations tried to elucidate possible underlying mechanisms and focused on genetic risk factors, neural correlates in neuroimaging studies and comprehensive neurocognitive characterisations.
10.2.2.3 Genetic Disposition
As mentioned in Sect. 10.1.1, efforts to elucidate pathomechanisms in psychiatry often focus on the interaction of both genetic and environmental factors. Applied to the co-occurrence of OCS and schizophrenia, SGA treatment can be perceived as an environmental factor possibly acting on the background of a primary genetic predisposition (see Chap. 8). To investigate this hypothesis, a South Korean research group evaluated association of the glutamate transporter gene SLC1A1 and second-onset OCS during treatment with SGAs (Kwon et al. 2009). Within primary OCD, several independent studies have consistently reported disease risk associated with SNPs in this gene (Veenstra-VanderWeele et al. 2001) (Arnold et al. 2006; Dickel et al. 2006; Shugart et al. 2009; Stewart et al. 2007; Wendland et al. 2009). Kwon et al. hypothesised that genetic variations in the candidate gene SLC1A1 modulate the susceptibility to develop OCS during SGA treatment (Kwon et al. 2009). Accordingly, they found a strong association (odds ratio of 3.96) with the A/C/G dominant haplotype rs2228622/rs3780413/rs37801412. However, a study investigating 103 schizophrenia patients of European descent could not replicate these findings (Schirmbeck et al. 2012a). Meanwhile additional genetic risk factors have been proposed: Kwon et al. described a genetic interaction of the SLC1A1 polymorphism with variants in the gene DLGAP3 (discs large-associated protein 3) and an association with SGA-induced OCS (Ryu et al. 2011). In a Chinese sample, an interaction of SNPs in SLC1A1 and the type 2B subunit of the N-methyl-D-aspartate receptor gene (GRIN2B) was reported (Cai et al. 2013). Based on neurotrophic theories, an association between the Val66Met polymorphism in the gene BDNF (brain-derived neurotrophic factor) and OCS in schizophrenia has been proposed (Hashim et al. 2012). So far, findings regarding BDNF, DLGAP3 and GRIN2B have not been replicated in independent studies. Future studies investigating GxEIs in the development of second-onset OCS should include additional environmental factors such as psychosocial stressors (e.g. critical life events, interpersonal relations, changes of the vocational situation or the present state of general physical health) (Schirmbeck and Zink 2013b).
10.2.2.4 Neurocognitive Correlates of SGA-Induced OCS
A set of neurocognitive deficits have been consistently linked to primary OCD, such as impaired cognitive shifting abilities, inhibitory control and effective planning strategies (Kuelz et al. 2004). In accordance, associations between cognitive impairment and comorbid OCS have been thoroughly investigated in patients with schizophrenia (Cunill et al. 2013; Lysaker and Whitney 2009) (see Chap. 7). Attempts to differentiate between schizophrenia patients with and without OCS resulted in somewhat inconclusive findings. Several authors did not find any significant differences (Achim et al. 2011; Hermesh et al. 2003; Meijer et al. 2013; Öngür and Goff 2005; Tiryaki and Ozkorumak 2010; Tumkaya et al. 2009; Whitney et al. 2004), others even reported better cognitive abilities (Borkowska et al. 2013; Lee et al. 2009), especially during ARMS (Zink et al. 2014a). Most publications, however, documented more pronounced deficits in processing speed (Michalopoulou et al. 2014), executive functioning (Hwang et al. 2000; Lysaker et al. 2002, 2009), cognitive flexibility (Kumbhani et al. 2010; Patel et al. 2010) and also delayed visual memory (Berman et al. 1998; Schirmbeck et al. 2011). The observed heterogeneity may partly be explained by the diverse clinical characteristics of investigated comorbid samples.
Only one study specifically focused on SGA-induced OCS and included a comprehensive set of OCD-related cognitive domains. At baseline (Schirmbeck et al. 2011) and 12 months later (Schirmbeck et al. 2012b), patients with comorbid OCS showed pronounced deficits in cognitive flexibility, visuospatial perception and visual memory, with increased between-group effect sizes over the 12-month assessment period. Performance in these domains correlated with OCS severity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


