Effects of ovariectomy and estrogen or androgen treatment on vaginal tissue structure (a–d). Female rats were left intact (control; a) or ovariectomized and after 2 weeks were infused with vehicle (b) or testosterone (c) or testosterone and estradiol (d). Hormone treatment was maintained for 2 weeks. Vaginal tissue was fixed, sectioned, and subjected to Masson’s trichrome staining as described in references 19 and 26.
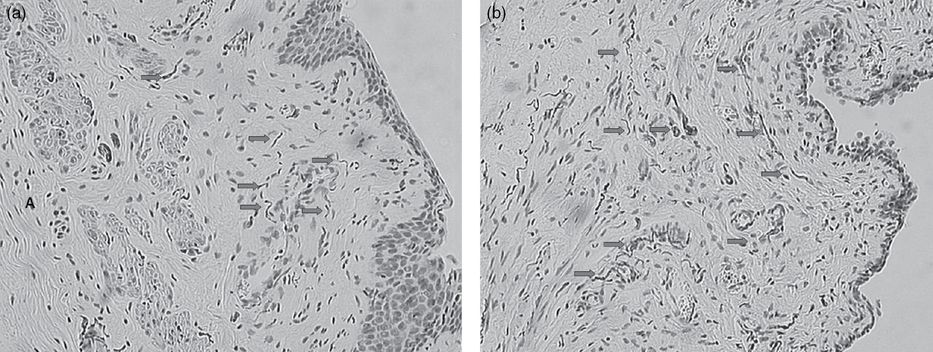
Effects of ovariectomy and androgen treatment on vaginal tissue nerve fiber network density. Female rats were left intact (control; a) or ovariectomized and after 2 weeks were infused with testosterone (b). Hormone treatment was started 2 weeks after ovariectomy and was maintained for 2 weeks. Vaginal tissue was fixed and paraffin-embedded and vaginal tissue sections subjected to immunostaining procedures using anti-PGP 9.5 primary antibody and counterstained with Gill’s hematoxylin. In vaginal tissue from intact rats (a), nerve fibers extending from the lamina propria into the epithelial layer were occasionally observed – see arrows in (a). PGP-positive fibers were commonly observed surrounding blood vessels (arrows) as depicted in tissue sections from intact control (a) and testosterone-infused (11 µg/day for 14 days), ovariectomized animals (b). Details are given in references 19 and 26. A black and white version of this figure will appear in some formats. For the color version, please refer to the plate section.
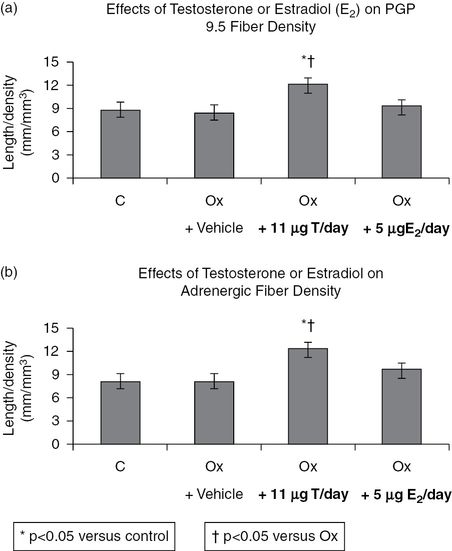
Effects of ovariectomy (Ox), and testosterone (T) or estradiol (E2) on PGP 9.5 density (a) and adrenergic fiber length-density (b). Rats were ovariectomized for 2 weeks, followed by 2 weeks of continuous infusion of vehicle or hormone replacement using subcutaneous osmotic pumps. Animals were sacrificed and vaginal tissue was removed, fixed, and embedded as described above. Immunostaining with the appropriate antibody to PGP 9.5 or tyrosine hydroxylase (a marker for the adrenergic nerve fiber) was employed. The density of the nerve fibers was assessed as described in reference 19. *P <0.001 versus control, †P <0.01 versus Ox.
Pelletier et al. [20] reported that ovariectomy produced significant reduction in the thickness of the lamina propria area and treatment with DHEA restored the thickness to approximately 69% of the intact value. In the ovariectomized animals, protein gene product 9.5 (PGP 9.5)-immunopositive fibers and muscularis layer thickness were reduced. These reductions were prevented with DHEA treatment. In addition, DHEA treatment resulted in increased density of TH-containing nerve fibers in the vagina. These findings paralleled those reported by us [19] and suggest that DHEA is acting as an androgen or androgen precursor in the vagina.
As in the prenatal and early postnatal periods, androgens are known to modulate clitoral growth in adolescents and adults. This is supported by clinical observations in women with disorders of sex development. Mutations in steroidogenic factor NR5A1 in patients with 46 XY genotype often produce sufficient testosterone for spontaneous virilization during puberty. Phenotypic females with NR5A1 mutations may present with clitoromegaly at puberty [21]. In another study, female-to-male transsexuals treated with testosterone exhibited signs of polycystic ovarian disease, but no noticeable endometrial pathologies. However, increases in clitoral size, libido, body, and facial hair, deepened voices, and decline in breast size were all noted [22,23]. In utero, virilization of the fetus can occur due to loss of activity of 17α-hydroxylase and 21-hydroxylases that shift the pathway to androgen biosynthesis by the adrenal gland, resulting in clitoral growth.
Androgens regulate key vasomotor and trophic enzymes in genital tissue
Blood flow in the vagina is critical for vaginal engorgement. This hemodynamic process is thought to be important for vaginal sexual arousal. Nitric oxide (NO), one of the key mediators of vaginal blood flow, is thought to be the non-adrenergic non-cholinergic neurotransmitter and the vasoactive dilator. In female rats, Kim et al. [24,25] demonstrated that administration of inhibitors of nitric oxide synthase (NOS) and inhibitors of guanylyl cyclase diminished vaginal blood flow in response to pelvic nerve stimulation. These observations implicate NO/cGMP as one of the key mediators of vaginal smooth muscle relaxation and pelvic nerve-stimulated increase in blood flow [26]. Treatment of ovariectomized rabbits with testosterone resulted in increased expression and activity of total NOS in the proximal vagina, but not in the distal vagina [27]. Treatment with 5α dihydrotestosterone (5α-DHT) resulted in increased expression and activity of total NOS in both the proximal and distal vagina.
Arginase hydrolyzes arginine, the main co-substrate for NOS [28]. Treatment of ovariectomized animals with DHEA, ∆5 A-diol, or 5α-DHT resulted in decreased arginase activity in the proximal and distal vagina. Interestingly, treatment with testosterone increased arginase activity in the proximal vagina, but not in the distal vagina. These observations suggest that, in vaginal tissue, arginase activity is modulated by androgens in a tissue- and region-specific manner [27]. ∆5 A-diol produced effects similar to those obtained with 5α-DHT. These observations indicate that ∆5 A-diol acted specifically as an androgen in the rabbit vagina.
Androgens modulate female genital sexual arousal responses
Peripheral sexual arousal consists of increased pelvic blood flow that causes genital vasocongestion and production of plasma transudate and mucin in the vagina. These vascular events are also accompanied by increased sensitivity of afferent nerves and changes in the tissue properties of the vaginal canal and the clitoris, among other genital tissues. Thus, genital sexual responses are in part regulated by the vascular smooth muscle in the vaginal blood vessels of the submucosa and the clitoral corpora cavernosa, as well as the tone of the non-vascular smooth muscle within the vaginal muscularis. Androgens may play an important role in vaginal hemodynamics and in maintaining the vaginal sexual arousal response. Vaginal blood flow in response to pelvic nerve stimulation in the animal model was significantly diminished in ovariectomized rats infused with vehicle, while testosterone treatment of ovariectomized animals enhanced vaginal blood flow responses (Figures 10.4 and 10.5) [29]. Given the histological and biochemical data presented in the previous sections, it is likely that neuronal structure/function, smooth muscle health, and expression of NOS and arginase provide a mechanistic basis for the effects of androgens on vaginal blood flow.
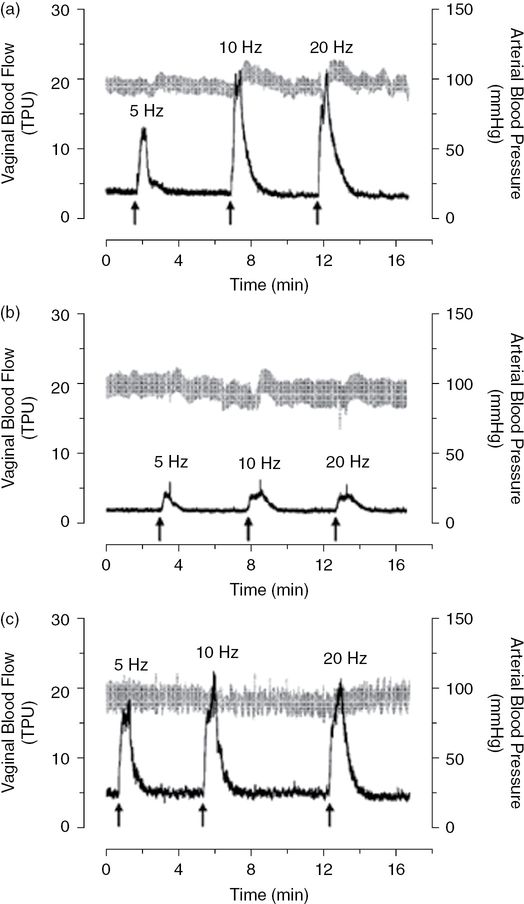
Vaginal blood flow recordings. After 2 weeks of vehicle or testosterone infusion, vaginal blood flow in response to pelvic nerve stimulation at varying frequencies was assessed by laser Doppler flowmetry (black). Systemic blood pressure was recorded simultaneously (gray). Shown are representative vaginal blood flow recordings from intact control (a) and ovariectomized rats infused with vehicle (b) or 55 µg/day of testosterone (c). Data derived from such recordings were then subjected to statistical analyses, as shown in Figure 10.5. TPU, tissue perfusion units. (With permission from reference 29.)
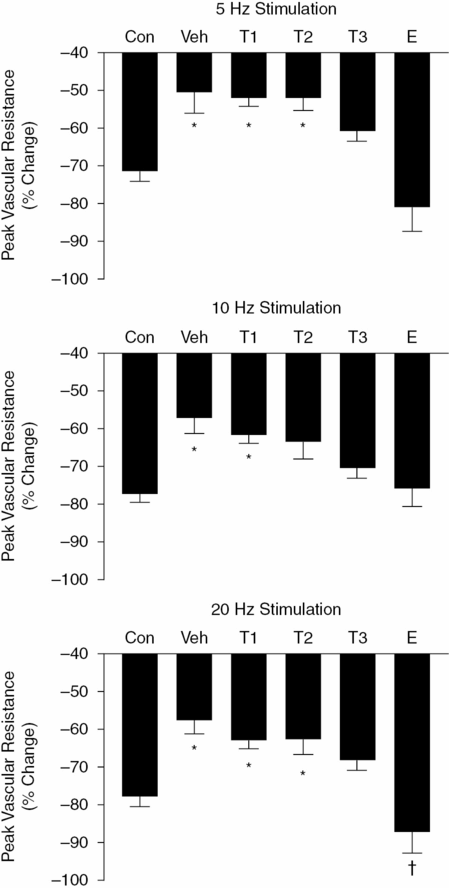
Effect of testosterone (T) on vaginal vascular resistance. Instantaneous vascular resistance values were calculated from vaginal blood flow and systemic blood pressure data (see Figure 10.4). The percent increase in mean peak vascular resistance over baseline vascular resistance was determined for each animal. For comparison, data from ovariectomized rats infused with a physiological dose of estradiol (E; 15 µg/day, resulting in a plasma concentration of 31 ± pg/mL) have been included. These data were derived from a previous study regarding the effects of estradiol on vaginal blood flow. Values are the mean ± SEM for each treatment group (see Figure 10.1). For each frequency of pelvic nerve stimulation, comparisons between groups were analyzed by one-way analysis of variance (ANOVA) and Tukey’s post hoc test (*P <0.05 vs. control group; †P <0.05 vs. group T3). (With permission from reference 29.)
In studies with ovariectomized animals, atrophy and inflammatory changes were noted in the vagina. DHEA, an androgen precursor, stimulated vaginal epithelium growth with mucous cells typical of an androgenic effect, produced epithelium mucification, and increased muscularis thickness [30,31]. Interestingly, treatment of ovariectomized animals with ∆5 A-diol increased total NOS activity in proximal and distal vagina, similar to that obtained with 5α-DHT [27]. These observations suggest that ∆5A-diol may be a unique hormone with specific activity in the vagina [32–34] or simply conversion into 5α-DHT, and modulates NOS activity in the vagina via an androgenic mechanism.
It has been suggested that androgens are important in vaginal mucification [34,35]. While moisture production for lubrication is thought to be estrogen dependent by increasing the moisture content in the vagina, androgens are thought to be important for the production of mucin. Lubrication encompasses secretion of sialo-glycoproteins, which provide the lubricant substance, and is modulated by androgens. Vaginal mucin production, as assessed by tissue sialic acid content, has been reported to be androgen dependent. Testosterone treatment of ovariectomized rabbits restored sialic acid to that of the vehicle-treated group (Traish et al., unpublished data). These data are consistent with the findings of Kennedy and Armstrong, who have shown that androgens increase vaginal mucification in the animal model [34,35]. In a separate study, treatment of ovariectomized rats with topical DHEA resulted in complete reversal of vaginal atrophy and stimulated proliferation and mucification of the vaginal epithelium [36]. Furthermore, DHEA treatment resulted in vaginal epithelial mucification [31,32].
Testosterone has also been shown to regulate vaginal muscularis function. Ovariectomy reduces vaginal muscularis relaxation to electric field stimulation and to exogenous vasointestinal polypeptide (VIP) in organ bath studies [37]. Estrogen treatment of animals failed to improve the relaxation response to either electrical stimulation or VIP. In contrast, testosterone treatment enhanced relaxation to electrical stimulation and restored VIP-induced relaxation, suggesting that androgens may modulate neurotransmitter function. Further, 5α-DHT, ∆5A-diol, and DHEA had similar effects to that of testosterone, indicating that several androgen hormones are equally effective in facilitating neurogenic or VIP-induced relaxation. These observations may be explained by increased neurogenic input or upregulation of neurotransmitter receptors mediating relaxation. Although the main neurotransmitter mediating relaxation of the rabbit vaginal muscularis is yet to be identified [37], similar mechanisms have been demonstrated in other tissues with VIP. Taken together, these data suggest that androgens facilitate vaginal smooth muscle relaxation, while estrogens attenuate this response [37]. In addition, tissue from testosterone-infused ovariectomized animals developed significantly greater contractile force to norepinephrine, suggesting that testosterone may be an important regulator of vaginal non-vascular smooth muscle contractility. It must be noted that these studies were carried out with supraphysiological levels of hormones, so this may be a pharmacologic rather than physiologic effect. Yet, Labrie et al. [38,39] recently provided clinical data that intravaginal DHEA improved vaginal sexual arousal and had beneficial effects on orgasm in postmenopausal women.
Clinical implications of androgen deficiency or excess in female genital physiology
Androgen excess
Congenital adrenal hyperplasia (CAH)
Conditions of CAH are generally diagnosed by neonatologists and pediatricians and are not pertinent, with limited exceptions, to the practice of the adult office gynecologist. Therefore, the treatment of these conditions will not be considered in this short treatise.
Polycystic ovary syndrome (PCOS)
PCOS is a pertinent exception to the group of disorders that are congenital in origin, because gynecologists will see these conditions quite regularly in the office. Approximately 10% of women are thought to exhibit androgen excess in adolescence and/or in adulthood. The excess androgens have numerous effects on the female genital tract. The ovaries are large and thickened and may present with pelvic discomfort. Women will present with hirsutism and acne, but also with menstrual irregularity and infertility. Of serious concern is the effect of prolonged amenorrhea on endometrial hyperplasia and the risk of endometrial cancer [40].
Although there is no direct effect on the female genital tract, practicing gynecologists should be aware that PCOS is linked to increased cardiovascular (CV) risk with considerable disagreements among studies as to the actual increase in CV events. Treatment of women with PCOS and infertility still may involve the use of clomiphene stimulation, wedge resection of the ovaries by a variety of techniques, and more sophisticated gonadotropin stimulation of the ovaries. Younger women present to gynecologists for hirsutism, acne, seborrhea, and often with concurrent menstrual disorders. Often, estrogen-progestin therapies are used to lower androgen levels by directly suppressing the mid-cycle surge of androgens, along with raising sex hormone-binding globulin (SHBG) levels to decrease free testosterone [41,42].
Androgen deficiency
One of the main problems with diagnosing androgen deficiency is that women produce about one-tenth of the amount of testosterone produced by men. Although free testosterone assays are the most accurate (measured in picogram amounts), they have been developed for men and the accuracy of the results is questioned in women. Despite this, it was thought that the current assays were adequate for the diagnosis of androgen deficiency. One approach is the measurement of DHEA-S, a main precursor to testosterone, and the main precursor in postmenopausal women. DHEA is the most abundant steroid produced by men and women, and the measurement is more accurate, as it is reported in microgram quantities. Measuring DHEA-S along with free testosterone lessens the potential decreased accuracy of the free testosterone assay. Davison et al. [43] investigated the levels of androgens in 1400 women from ages 18 to 75 years, with self-reported normal sexual function, and presented data listed by decade, along with a differentiation of women with normal and surgical menopause. These data (Table 10.1) provide the most comprehensive baseline level of androgens in women to date [44].
18–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–75 years | |
|---|---|---|---|---|---|---|
Total T (nmol/L) | ||||||
Mean | 1.58 | 1.11 | 0.92 | 0.81 | 0.66 (0.38 SM) | 0.71 (0.39 SM) |
10th percentile | 0.86 | 0.58 | 0.50 | 0.40 | 0.20 | 0.30 |
90th percentile | 2.47 | 1.70 | 1.40 | 1.30 | 1.25 | 1.10 |
cFree T (pmol/L) | ||||||
Mean | 23.61 | 17.25 | 13.67 | 11.62 | 10.81 (5.54 SM) | 9.76 (6.06 SM) |
10th percentile | 12.91 | 8.17 | 5.80 | 5.25 | 3.69 | 3.43 |
90th percentile | 38.64 | 31.70 | 23.52 | 21.28 | 20.88 | 17.26 |
DHEA-S (µmol/L) | ||||||
Mean | 7.49 | 4.72 | 4.31 | 3.42 | 2.36 (1.89 SM) | 1.76 (1.13 SM) |
10th percentile | 4.03 | 2.20 | 1.86 | 1.30 | 0.70 | 0.50 |
90th percentile | 10.78 | 7.90 | 7.31 | 6.20 | 5.30 | 3.10 |
A-dione (nmol/L) | ||||||
Mean | 8.46 | 6.44 | 5.15 | 4.17 | 3.14 (2.15 SM) | 3.07 (2.93 SM) |
10th percentile | 4.86 | 3.00 | 2.64 | 2.00 | 1.30 | 1.30 |
90th percentile | 13.72 | 9.46 | 8.48 | 6.29 | 5.10 | 5.40 |
cFree T, calculated free T; SM, surgical menopause; A-dione, androstenedione;
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


