Classification
Nearly 95 percent of ectopic pregnancies are implanted in the various segments of the fallopian tube and give rise to fimbrial, ampullary, isthmic, or interstitial tubal pregnancies (Fig. 19-1). As shown, the ampulla is the most frequent site, followed by the isthmus. The remaining 5 percent of nontubal ectopic pregnancies implant in the ovary, peritoneal cavity, cervix, or prior cesarean scar. Occasionally, a multifetal pregnancy is composed of one conceptus with normal uterine implantation coexisting with one implanted ectopically. The natural incidence of these heterotopic pregnancies approximates 1 per 30,000 pregnancies. However, because of assisted reproductive technologies (ART), their incidence has increased to 1 in 7000 overall, and following ovulation induction, it may be as high as 0.5 to 1 percent (Mukul, 2007). Rarely, twin tubal pregnancy with both embryos in the same tube or with one in each tube has been reported (Eze, 2012; Svirsky, 2010).
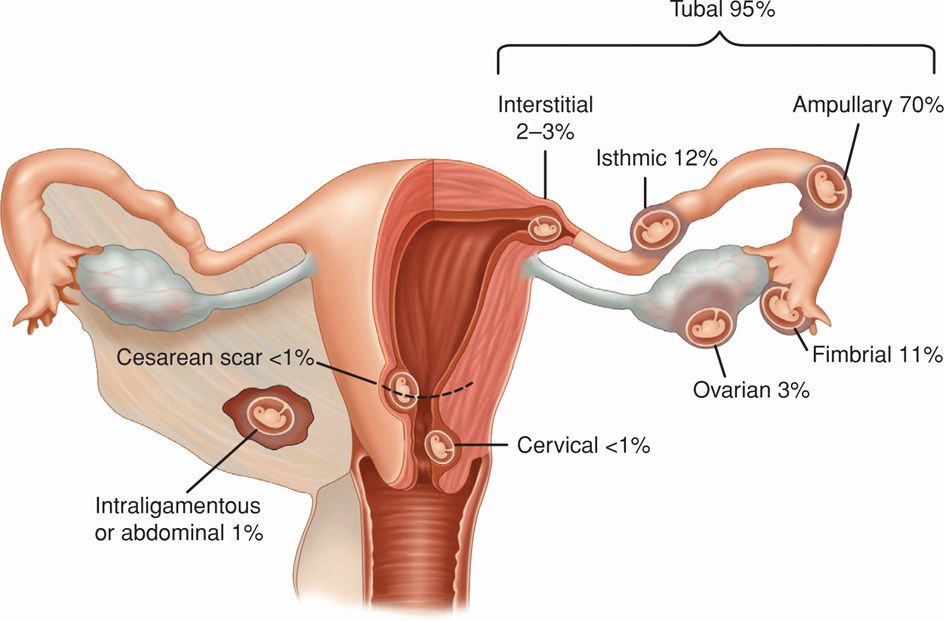
FIGURE 19-1 Sites of implantation of 1800 ectopic pregnancies from a 10-year population-based study. (Data from Callen, 2000; Bouyer, 2003.)
Regardless of location, D-negative women with an ectopic pregnancy who are not sensitized to D-antigen should be given IgG anti-D immunoglobulin (American College of Obstetricians and Gynecologists, 2013). In first-trimester pregnancies, a 50-μg or a 300-μg dose is appropriate, whereas a standard 300-μg dose is used for later gestations.
 Risks
Risks
Abnormal fallopian tube anatomy underlies many cases of tubal ectopic pregnancy. Surgeries for a prior tubal pregnancy, for fertility restoration, or for sterilization confer the highest risk of tubal implantation. After one previous ectopic pregnancy, the chance of another approximates 10 percent (Ankum, 1996; Skjeldestad, 1998). Prior sexually transmitted disease or other tubal infection, which can distort normal tubal anatomy, is another common risk factor. Specifically, one episode of salpingitis can be followed by a subsequent ectopic pregnancy in up to 9 percent of women (Westrom, 1992). Similarly, peritubal adhesions subsequent to salpingitis, appendicitis, or endometriosis may increase the risk for tubal pregnancy. Salpingitis isthmica nodosa, which is a condition in which epithelium-lined diverticula extend into a hypertrophied muscularis layer, also poses an increased risk (Schippert, 2012). Congenital fallopian tube anomalies, especially those secondary to in utero diethylstilbestrol exposure, can also lead to malformed tubes and higher ectopic rates (Hoover, 2011).
Infertility, per se, as well as the use of ART to overcome it, is linked to substantively increased risks for ectopic pregnancy (Clayton, 2006). And “atypical” implantations—cornual, abdominal, cervical, ovarian, and heterotopic pregnancy—are more common following ART procedures. Smoking is also a known association, although the underlying mechanism is unclear (Waylen, 2009). Last, with any form of contraception, the absolute number of ectopic pregnancies is decreased because pregnancy occurs less often. However, with some contraceptive method failures, the relative number of ectopic pregnancies is increased. Examples include tubal sterilization, copper and progestin-releasing intrauterine devices (IUDs), and progestin-only contraceptives (Furlong, 2002).
 Evolution and Potential Outcomes
Evolution and Potential Outcomes
With tubal pregnancy, because the fallopian tube lacks a submucosal layer, the fertilized ovum promptly burrows through the epithelium. The zygote comes to lie near or within the muscularis, which is invaded in most cases by rapidly proliferating trophoblast. The embryo or fetus in an ectopic pregnancy is often absent or stunted.
Outcomes of ectopic pregnancy include tubal rupture, tubal abortion, or pregnancy failure with resolution. With rupture, the invading expanding products of conception and associated hemorrhage may tear rents in the fallopian tube at any of several sites. As a rule, if the tube ruptures in the first few weeks, the pregnancy is most likely located in the isthmic portion, whereas the ampulla is slightly more distensible (Fig. 19-2). However, if the fertilized ovum implants within the interstitial portion, rupture usually occurs later. Tubal ectopic pregnancies usually burst spontaneously but may occasionally rupture following coitus or bimanual examination.
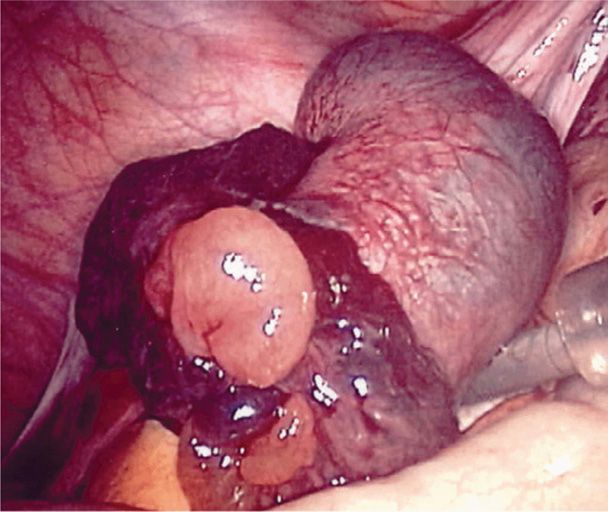
FIGURE 19-2 Ruptured ampullary early tubal pregnancy. (Photograph contributed by Dr. Togas Tulandi.)
Alternatively, the pregnancy may abort out the distal fallopian tube, and the frequency of this depends in part on the initial implantation site. Abortion is common in fimbrial and ampullary pregnancies, whereas rupture is the usual outcome with those in the tubal isthmus. With tubal abortion, hemorrhage disrupts the connection between the placenta and membranes and the tubal wall. If placental separation is complete, the entire conceptus may be extruded through the fimbriated end into the peritoneal cavity. At this point, hemorrhage may cease and symptoms eventually disappear. Some bleeding usually persists as long as products remain in the tube. Blood slowly trickles from the tubal fimbria into the peritoneal cavity and typically pools in the rectouterine cul-de-sac. If the fimbriated extremity is occluded, the fallopian tube may gradually become distended by blood, forming a hematosalpinx.
Last, an unknown number of ectopic pregnancies spontaneously fail and are reabsorbed. This may be documented now more regularly with the advent of sensitive β-hCG assays.
There are differences between “acute” ectopic pregnancy just described and “chronic” ectopic pregnancy. The more common acute ectopic pregnancies are those with a high serum β-hCG level and rapid growth, leading to an immediate diagnosis. These carry a higher risk of tubal rupture (Barnhart, 2003c). With chronic ectopic pregnancy, abnormal trophoblast die early, and thus negative or lower, static serum β-hCG levels are found (Brennan, 2000). Chronic ectopic pregnancies typically rupture late, if at all, but commonly form a complex pelvic mass, which often is the reason prompting diagnostic surgery (Cole, 1982; Grynberg, 2009; Uğur, 1996).
 Clinical Manifestations
Clinical Manifestations
Earlier patient presentation and more precise diagnostic technology typically allow identification before rupture. In these cases, symptoms and signs of ectopic pregnancy are often subtle or even absent. The woman does not suspect tubal pregnancy and assumes that she has a normal early pregnancy or is having a miscarriage.
With later diagnosis, a “classic” presentation is characterized by the triad of delayed menstruation, pain, and vaginal bleeding or spotting. With tubal rupture, there is usually severe lower abdominal and pelvic pain that is frequently described as sharp, stabbing, or tearing. There is tenderness during abdominal palpation. Bimanual pelvic examination, especially cervical motion, causes exquisite pain. The posterior vaginal fornix may bulge from blood in the rectouterine cul-de-sac, or a tender, boggy mass may be felt to one side of the uterus. Although minimal early, later the uterus may be pushed to one side by an ectopic mass. The uterus may also be slightly enlarged due to hormonal stimulation. Symptoms of diaphragmatic irritation, characterized by pain in the neck or shoulder, especially on inspiration, develop in perhaps half of women with sizable hemoperitoneum.
Some degree of vaginal spotting or bleeding is reported by 60 to 80 percent of women with tubal pregnancy. Although profuse vaginal bleeding is suggestive of an incomplete abortion, such bleeding occasionally is seen with tubal gestations. Moreover, tubal pregnancy can lead to significant intraabdominal hemorrhage. Responses to moderate bleeding include no change in vital signs, a slight rise in blood pressure, or a vasovagal response with bradycardia and hypotension. Birkhahn and colleagues (2003) noted that in 25 women with ruptured ectopic pregnancy, most at presentation had a heart rate < 100 beats per minute and a systolic blood pressure > 100 mm Hg. Blood pressure will fall and pulse will rise only if bleeding continues and hypovolemia becomes significant. Vasomotor disturbances develop, ranging from vertigo to syncope.
Even after substantive hemorrhage, hemoglobin or hematocrit readings may at first show only a slight reduction. Hence, after an acute hemorrhage, a decline in hemoglobin or hematocrit level over several hours is a more valuable index of blood loss than is the initial level. In approximately half of women with a ruptured ectopic pregnancy, varying degrees of leukocytosis up to 30,000/μL may be documented.
Decidua is endometrium that is hormonally prepared for pregnancy, and the degree to which the endometrium is converted with ectopic pregnancy is variable. Thus, in addition to bleeding, women with ectopic tubal pregnancy may pass a decidual cast, which is the entire sloughed endometrium that takes the form of the endometrial cavity (Fig. 19-3). Importantly, decidual sloughing may also occur with uterine abortion. Thus, tissue should be carefully evaluated visually and then histologically for evidence of a conceptus. If no clear gestational sac is visually seen or if no villi are identified histologically within the cast, then the possibility of ectopic pregnancy must still be considered.
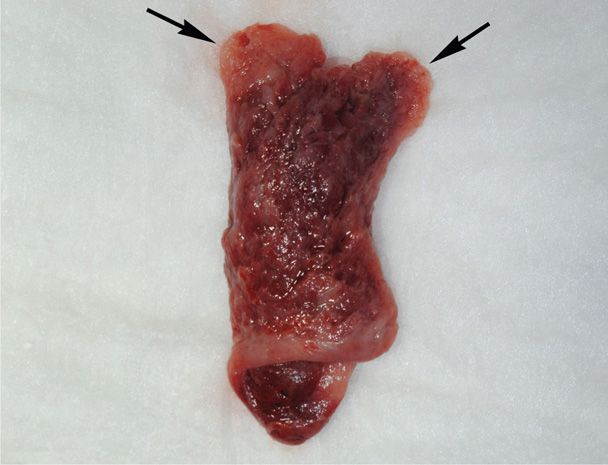
FIGURE 19-3 This decidual cast was passed by a patient with a tubal ectopic pregnancy. The cast mirrors the shape of the endometrial cavity, and each arrow marks the portion of decidua that lined the cornua.
 Multimodality Diagnosis
Multimodality Diagnosis
The differential diagnosis for abdominal pain coexistent with pregnancy is extensive. Pain may derive from uterine conditions such as miscarriage, infection, degenerating or enlarging leiomyomas, molar pregnancy, or round-ligament pain. Adnexal disease may include ectopic pregnancy; hemorrhagic, ruptured, or torsed ovarian masses; salpingitis; or tuboovarian abscess. Last, appendicitis, cystitis, renal stone, or gastroenteritis may be nongynecological sources of lower abdominal pain in early pregnancy.
A number of algorithms have been proposed to identify ectopic pregnancy. Most include these key components: physical findings, transvaginal sonography (TVS), serum β-hCG level measurement—both the initial and the subsequent pattern of rise or decline, and diagnostic surgery, which includes uterine curettage, laparoscopy, and occasionally, laparotomy (Fig. 19-4). Algorithm use applies only to hemodynamically stable women—those with presumed rupture should undergo prompt surgical therapy. For a suspected unruptured ectopic pregnancy, all diagnostic strategies involve trade-offs. Strategies that maximize detection of ectopic pregnancy may result in termination of a normal pregnancy. Conversely, those that reduce the potential for normal pregnancy interruption will delay ectopic pregnancy diagnosis. Patient desires for the index pregnancy are also discussed and may influence these trade-offs.
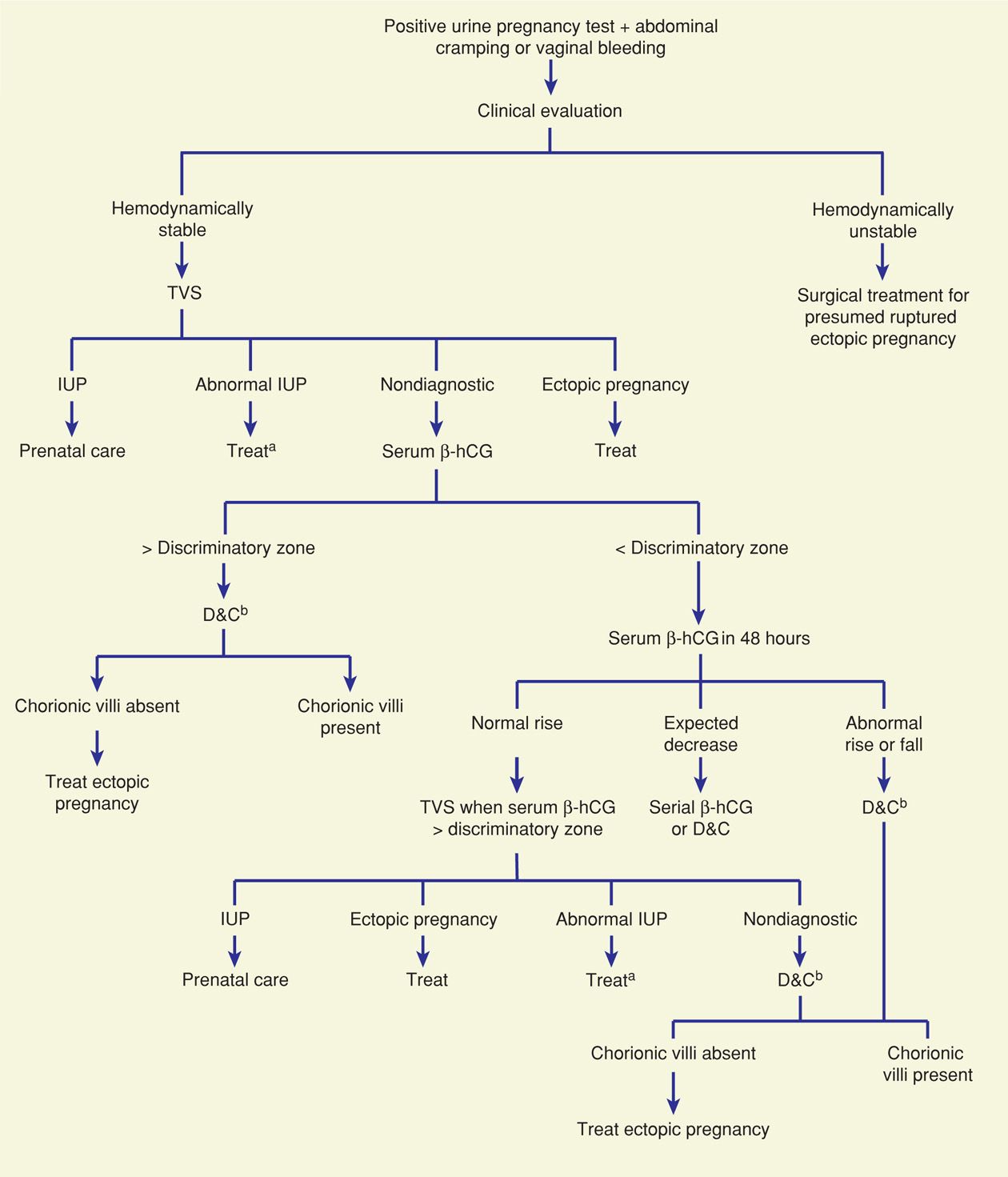
FIGURE 19-4 One suggested algorithm for evaluation of a woman with a suspected ectopic pregnancy. aExpectant management, D&C, or medical regimens are suitable options. bSerial serum β-hCG levels may be appropriate if a normal uterine pregnancy or if completed abortion is suspected clinically. β-hCG = beta human chorionic gonadotropin; D&C = dilatation and curettage; IUP = intrauterine pregnancy; TVS = transvaginal sonography. (Modified from Gala, 2012.)
Beta Human Chorionic Gonadotropin
Rapid and accurate determination of pregnancy is essential to identify an ectopic pregnancy. Current serum and urine pregnancy tests that use enzyme-linked immunosorbent assays (ELISAs) for β-hCG are sensitive to levels of 10 to 20 mIU/mL and are positive in > 99 percent of ectopic pregnancies (Kalinski, 2002). Rare cases of chronic ectopic pregnancy, described earlier, with negative serum β-hCG assay results have been reported.
With bleeding or pain and a positive pregnancy test result, an initial TVS is typically performed to identify gestation location. If a yolk sac, embryo, or fetus is identified within the uterus or the adnexa, then a diagnosis can be made. In many cases however, TVS is nondiagnostic, and tubal pregnancy is still a possibility. In these cases in which neither intrauterine nor extrauterine pregnancy is identified, the term pregnancy of unknown location (PUL) is used until additional clinical information allows determination of pregnancy location.
Levels above the Discriminatory Zone. A number of investigators have described discriminatory β-hCG levels above which failure to visualize an intrauterine pregnancy (IUP) indicates that the pregnancy either is not alive or is ectopic. Barnhart and colleagues (1994) reported that an empty uterus with a serum β-hCG concentration ≥ 1500 mIU/mL was 100-percent accurate in excluding a live uterine pregnancy. Some institutions set their discriminatory threshold higher at ≥ 2000 mIU/mL. Moreover, Connolly and associates (2013) reported evidence to suggest an even higher threshold. They noted that with live uterine pregnancies, a gestational sac was seen 99 percent of the time with a discriminatory level of 3510 mIU/mL.
If the initial β-hCG level exceeds the set discriminatory level and no evidence for a uterine pregnancy is seen with TVS, then the diagnosis is narrowed in most cases to a failed uterine pregnancy, completed abortion, or an ectopic pregnancy. Early multifetal gestation also remains a possibility. If there is a suspicion in a stable patient that a PUL could be a normal pregnancy, it is prudent to continue expectant management with serial β-hCG level assessment to avoid harming an early normal pregnancy. If patient history or extruded uterine tissue suggests a completed abortion, then serial β-hCG levels will drop rapidly. Otherwise, curettage will distinguish an ectopic from a nonliving uterine pregnancy. Some do not recommend diagnostic curettage because it results in unnecessary surgical therapy (Barnhart, 2002). This is countered by concern for methotrexate toxicity if this drug is given erroneously to women with a presumed ectopic pregnancy.
Levels below the Discriminatory Zone. If the initial β-hCG level is below the set discriminatory value, pregnancy location is often not technically discernible with TVS. With these PULs, serial β-hCG level assays are done to identify patterns that indicate either a growing or failing uterine pregnancy. Levels that rise or fall outside these expected parameters increase the concern for ectopic pregnancy. Thus, appropriately selected women with a possible ectopic pregnancy, but whose initial β-hCG level is below the discriminatory threshold, are seen 2 days later for further evaluation. First, with early normal progressing uterine pregnancies, Kadar and Romero (1987) reported that mean doubling time for serum β-hCG levels was approximately 48 hours. The lowest normal value for this increase was 66 percent. Barnhart and coworkers (2004) reported a 53-percent 48-hour minimum rise with a 24-hour minimum rise of 24 percent. Seeber and associates (2006) used an even more conservative 35-percent 48-hour rise. Importantly, Silva and colleagues (2006) caution that a third of women with an ectopic pregnancy will have a 53-percent rise at 48 hours. They further reported that no single pattern characterizes ectopic pregnancy and that approximately half of ectopic pregnancies will show decreasing β-hCG levels, whereas the other half will have increasing levels.
With a failing intrauterine pregnancy, patterned rates of β-hCG level decline can also be anticipated. Rates of decline ranging between 21 and 35 percent are commonly used. As seen in Table 19-1, the percentage drop is greater if the initial β-hCG level is higher.
TABLE 19-1. Expected Minimum Percentage Decline of Initial Serum β-hCG Levels to Subsequently Drawn Values for Nonliving Pregnancies
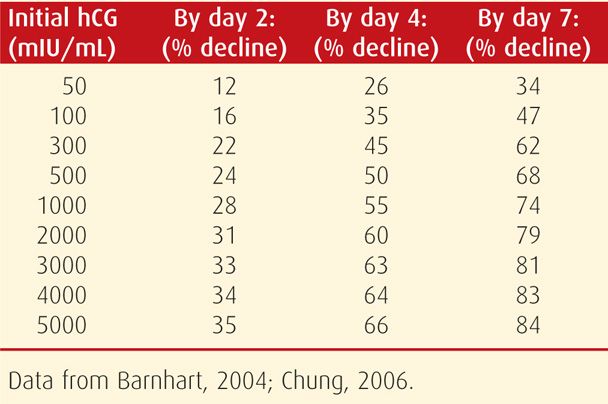
In pregnancies without these expected rises or falls in β-hCG levels, distinction between a nonliving intrauterine and an ectopic pregnancy may be aided by repeat β-hCG level evaluation (Zee, 2013). Also, uterine curettage is an option. Barnhart and associates (2003b) reported that endometrial biopsy was less sensitive than curettage. Before curettage, a second TVS examination may be indicated and may display new informative findings.
Serum Progesterone
A single serum progesterone measurement may clarify the diagnosis in a few cases (Stovall, 1989, 1992b). A value exceeding 25 ng/mL excludes ectopic pregnancy with 92.5-percent sensitivity (Lipscomb, 1999a; Pisarska, 1998). Conversely, values below 5 ng/mL are found in only 0.3 percent of normal pregnancies (Mol, 1998). Thus, values < 5 ng/mL suggest either a nonliving uterine pregnancy or an ectopic pregnancy. Because in most ectopic pregnancies, progesterone levels range between 10 and 25 ng/mL, the clinical utility is limited (American College of Obstetricians and Gynecologists, 2012). One caveat is that pregnancy achieved with ART may be associated with higher than usual progesterone levels (Perkins, 2000).
A number of preliminary studies have been done to evaluate novel markers to detect ectopic pregnancy (Rausch, 2012; Senapati, 2013). However, none of these are in current clinical use.
Transvaginal Sonography
Endometrial Findings. In a woman in whom ectopic pregnancy is suspected, TVS is performed to look for findings indicative of intrauterine or ectopic pregnancy. During endometrial cavity evaluation, an intrauterine gestational sac is usually visible between 4½ and 5 weeks. The yolk sac appears between 5 and 6 weeks, and a fetal pole with cardiac activity is first detected at 5½ to 6 weeks (Fig. 9-3, p. 170). With transabdominal sonography, these structures are visualized slightly later.
In contrast, with ectopic pregnancy, a trilaminar endometrial pattern can be diagnostic (Fig. 19-5). Its specificity is 94 percent, but with a sensitivity of only 38 percent (Hammoud, 2005). In addition, Moschos and Twickler (2008b) determined that in women with PUL at presentation, no normal pregnancies had a stripe thickness < 8 mm.
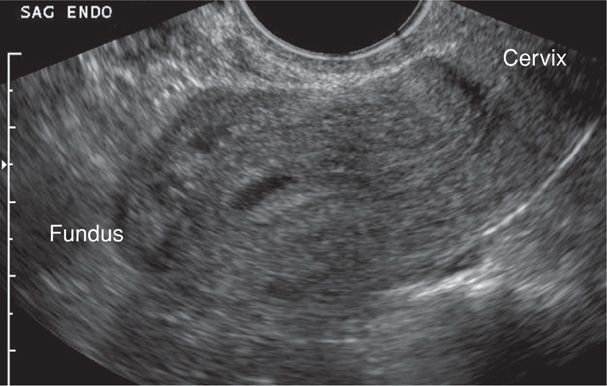
FIGURE 19-5 Transvaginal sonography of a pseudogestational sac within the endometrial cavity. Its cavity-conforming shape and central location are characteristic of these anechoic fluid collections. Distal to this fluid, the endometrial stripe has a trilaminar pattern, which is a common finding with ectopic pregnancy. (Image contributed by Dr. Elysia Moschos.)
Anechoic fluid collections, which might normally suggest an early intrauterine gestational sac, may also be seen with ectopic pregnancy. These include pseudogestational sac and decidual cyst. First, a pseudosac is a fluid collection between the endometrial layers and conforms to the cavity shape (see Fig. 19-5). If a pseudosac is noted, the risk of ectopic pregnancy is increased (Hill, 1990; Nyberg, 1987). Second, a decidual cyst is identified as an anechoic area lying within the endometrium but remote from the canal and often at the endometrial-myometrial border. Ackerman and colleagues (1993b) suggested that this finding represents early decidual breakdown and precedes decidual cast formation.
These two findings contrast with the intradecidual sign seen with intrauterine pregnancy. This is an early gestational sac and is eccentrically located within one of the endometrial stripe layers (Dashefsky, 1988). The American College of Obstetricians and Gynecologists (2011) advises caution in diagnosing a uterine pregnancy in the absence of a definite yolk sac or embryo.
Adnexal Findings. The sonographic diagnosis of ectopic pregnancy rests on visualization of an adnexal mass separate from the ovary (Fig. 19-6). If fallopian tubes and ovaries are visualized and an extrauterine yolk sac, embryo, or fetus is identified, then an ectopic pregnancy is clearly confirmed. In other cases, a hyperechoic halo or tubal ring surrounding an anechoic sac can be seen. Alternatively, an inhomogeneous complex adnexal mass is usually caused by hemorrhage within the ectopic sac or by an ectopic pregnancy that has ruptured into the tube. Overall, approximately 60 percent of ectopic pregnancies are seen as an inhomogeneous mass adjacent to the ovary; 20 percent appear as a hyperechoic ring; and 13 percent have an obvious gestational sac with a fetal pole (Condous, 2005). Importantly, not all adnexal masses represent an ectopic pregnancy, and integration of sonographic findings with other clinical information is necessary.
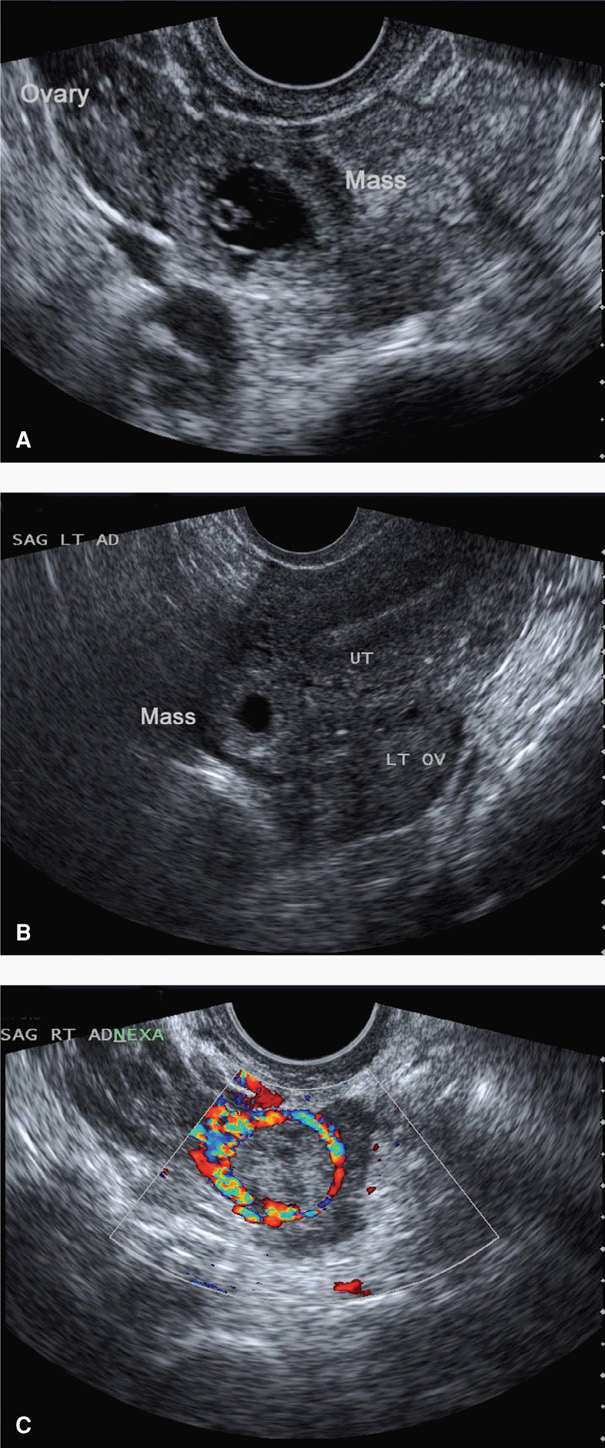
FIGURE 19-6 Various transvaginal sonographic findings with ectopic tubal pregnancies. For sonographic diagnosis, an ectopic mass should be seen in the adnexa separate from the ovary and may be seen as: (A) a yolk sac (shown here) and/or fetal pole with or without cardiac activity within an extrauterine sac, (B) an empty extrauterine sac with a hyperechoic ring, or (C) an inhomogeneous adnexal mass. In this last image, color Doppler shows a classic “ring of fire,” which reflects increased vascularity typical of ectopic pregnancies. LT OV = left ovary; SAG LT AD = sagittal left adnexal; UT = uterus.
Placental blood flow within the periphery of the complex adnexal mass—the ring of fire—can be seen with transvaginal color Doppler imaging. Although this can aid in the diagnosis, this finding can also be seen with a corpus luteum of pregnancy, and differentiation can be challenging.
Hemoperitoneum. In women with suspected ectopic pregnancy, evaluation for hemoperitoneum can add valuable clinical information. More commonly, this is completed using sonography, but assessment can also be made by culdocentesis. Sonographically, hemoperitoneum is anechoic or hypoechoic fluid. Blood initially collects in the dependent retrouterine cul-de-sac, and then additionally surrounds the uterus as it fills the pelvis (Fig. 19-7). As little as 50 mL can be seen in the cul-de-sac using TVS, and transabdominal imaging helps to assess the hemoperitoneum extent. For example, with significant intraabdominal hemorrhage, blood will track up the pericolic gutters to fill Morison pouch near the liver. Free fluid in this pouch typically is not seen until accumulated blood reaches 400 to 700 mL (Branney, 1995; Rodgerson, 2001; Rose, 2004). Diagnostically, peritoneal fluid in conjunction with an adnexal mass is highly predictive of ectopic pregnancy (Nyberg, 1991). Importantly, however, a small amount of peritoneal fluid is physiologically normal.
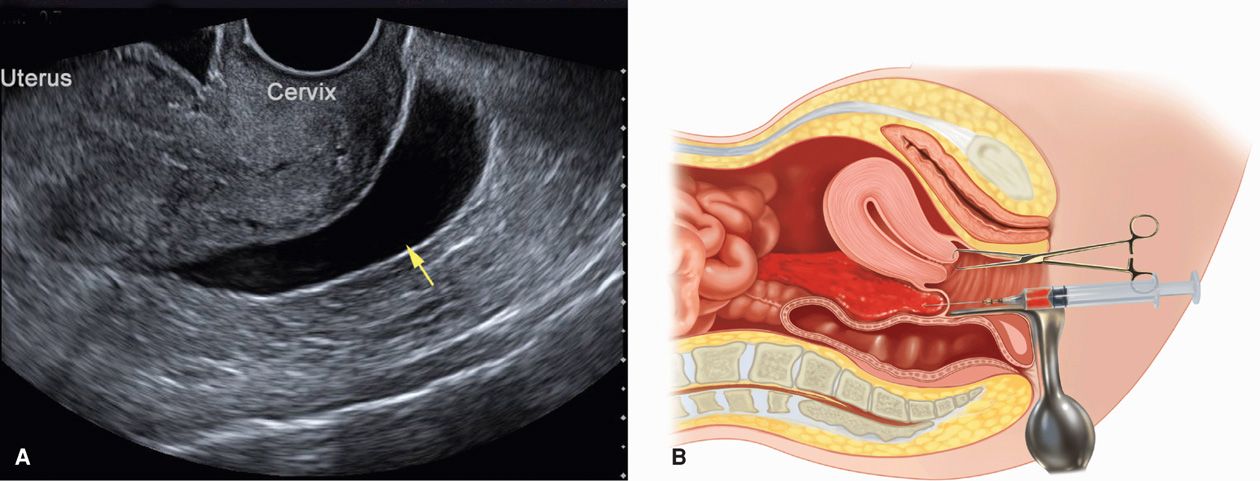
FIGURE 19-7 Techniques to identify hemoperitoneum. A. Transvaginal sonography of an anechoic fluid collection (arrow) in the retrouterine cul-de-sac. B. Culdocentesis: with a 16- to 18-gauge spinal needle attached to a syringe, the cul-de-sac is entered through the posterior vaginal fornix as upward traction is applied to the cervix with a tenaculum.
Culdocentesis is a simple technique used commonly in the past to identify hemoperitoneum. The cervix is pulled outward and upward toward the symphysis with a tenaculum, and a long 18-gauge needle is inserted through the posterior vaginal fornix into the retrouterine cul-de-sac. If present, fluid can be aspirated. However, a failure to do so is interpreted only as unsatisfactory entry into the cul-de-sac and does not exclude ectopic pregnancy. Fluid containing fragments of old clots or bloody fluid that does not clot is compatible with the diagnosis of hemoperitoneum. In contrast, if the blood sample clots, it may have been obtained from an adjacent blood vessel or from a briskly bleeding ectopic pregnancy. A number of studies have challenged its usefulness, and culdocentesis has been largely replaced by TVS (Glezerman, 1992; Vermesh, 1990).
Laparoscopy
Direct visualization of the fallopian tubes and pelvis by laparoscopy offers a reliable diagnosis in most cases of suspected ectopic pregnancy. There is also a ready transition to definitive operative therapy, which is discussed subsequently.
 Treatment Options
Treatment Options
Options for ectopic tubal pregnancy treatment include medical and surgical approaches, and their comparison is discussed on page 387. Medical therapy traditionally involves the antimetabolite methotrexate. Surgical choices include mainly salpingostomy or salpingectomy.
 Medical Management
Medical Management
Regimen Options
Methotrexate is a folic acid antagonist. It tightly binds to dihydrofolate reductase, blocking the reduction of dihydrofolate to tetrahydrofolate, which is the active form of folic acid. As a result, de novo purine and pyrimidine synthesis is halted, which leads to arrested DNA, RNA, and protein synthesis. Thus, methotrexate is highly effective against rapidly proliferating tissue such as trophoblast, and overall ectopic tubal pregnancy resolution rates approximate 90 percent with its use. However, bone marrow, gastrointestinal mucosa, and respiratory epithelium can also be harmed. It is directly toxic to hepatocytes and is renally excreted. Importantly, methotrexate is a potent teratogen, and methotrexate embryopathy is notable for craniofacial and skeletal abnormalities and fetal-growth restriction (Chap. 12, p. 248) (Nurmohamed, 2011). In addition, methotrexate is excreted into breast milk and may accumulate in neonatal tissues and interfere with neonatal cellular metabolism (American Academy of Pediatrics, 2001; Briggs, 2011). Based on all these findings, a list of contraindications and pretherapy laboratory testing is found in Table 19-2. Methotrexate is bound primarily to albumin, and its displacement by other medications such as phenytoin, tetracyclines, salicylates, and sulfonamides can increase methotrexate serum drug levels. Moreover, renal clearance of methotrexate may be impaired by nonsteroidal antiinflammatory drugs, probenecid, aspirin, or penicillins (Stika, 2012). Last, vitamins containing folic acid may lower methotrexate efficacy.
TABLE 19-2. Medical Treatment Protocols for Ectopic Pregnancy
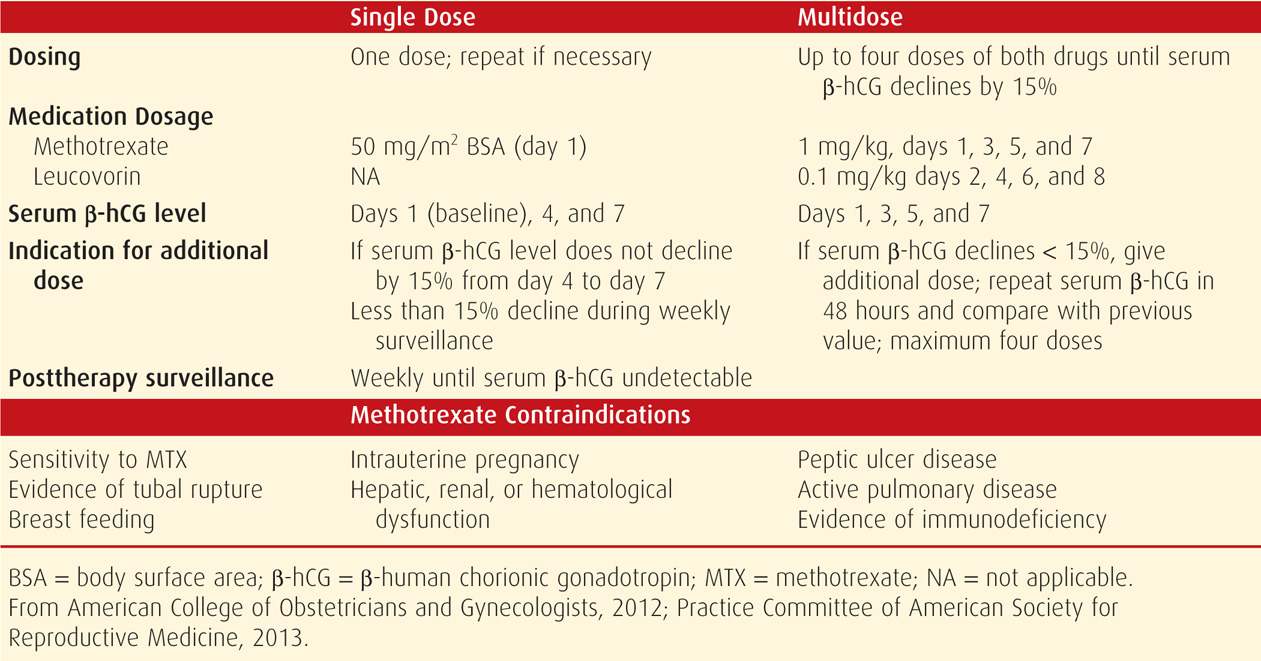
For ease and efficacy, intramuscular methotrexate administration is used most frequently for ectopic pregnancy resolution, and single-dose and multidose methotrexate protocols are available (see Table 19-2). As noted, methotrexate can lead to bone marrow depression. This toxicity can be blunted by early administration of leucovorin, which is folinic acid and has activity equivalent to folic acid. Thus, leucovorin, which is given within the multidose protocol, allows for some purine and pyrimidine synthesis to buffer side effects.
In comparing these two protocols, trade-offs are recognized. For example, single-dose therapy offers simplicity, less expense, and less intensive posttherapy monitoring and does not require leucovorin rescue. However, some but not all studies report a higher success rate for the multidose regimen (Alleyassin, 2006; Barnhart, 2003a; Lipscomb, 2005). At our institution, we use single-dose methotrexate.
A third hybrid “two dose” protocol has been proposed in an effort to balance the efficacy and convenience of the two most commonly used protocols (Barnhart, 2007). The regimen involves administering 50 mg/m2 of methotrexate on days 0 and 4 without leucovorin rescue. Data are limited on its comparative efficacy with the two standard regimens, but one study showed single-dose to be as effective as the two-dose regimen (Gungorduk, 2011).
Patient Selection
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. With medical therapy, some classic predictors of success include a low initial serum β-hCG level, small ectopic pregnancy size, and absent fetal cardiac activity. Of these, initial serum β-hCG level is the single best prognostic indicator of successful treatment with single-dose methotrexate. Specifically, reported failure rates are 1.5 percent if the initial serum β-hCG concentration is < 1000 mIU/mL; 5.6 percent with 1000 to 2000 mIU/mL; 3.8 percent with 2000 to 5000 mIU/mL; and 14.3 percent when levels are between 5000 and 10,000 mIU/mL (Menon, 2007). Interestingly, the initial serum β-hCG value is not a valid indicator of the number of doses needed for successful resolution (Nowak-Markwitz, 2009).
Many early trials also used “large size” as an exclusion criterion, although these data are less precise. Lipscomb and colleagues (1998) reported a 93-percent success rate with single-dose methotrexate when the ectopic mass was < 3.5 cm. This compared with success rates between 87 and 90 percent when the mass was > 3.5 cm. Last, most studies report increased failure rates if there is cardiac activity. Lipscomb and colleagues (1998) reported an 87-percent success rate in such cases.
Treatment Side Effects
These regimens are associated with minimal laboratory changes and symptoms, although occasional toxicity may be severe. Kooi and Kock (1992) reviewed 16 studies and reported that adverse effects were resolved by 3 to 4 days after methotrexate was discontinued. The most common were liver involvement—12 percent, stomatitis—6 percent, and gastroenteritis—1 percent. One woman had bone marrow depression. Regarding long-term effects, Oriol and coworkers (2008), using antimüllerian hormone assays, concluded that ovarian reserve was not compromised by single-dose methotrexate therapy.
Importantly, 65 to 75 percent of women initially given methotrexate will have increasing pain beginning several days after therapy. This separation pain generally is mild and relieved by analgesics. In a series of 258 methotrexate-treated women by Lipscomb and colleagues (1999b), 20 percent had pain severe enough to require evaluation in the clinic or emergency room. Ultimately, 10 of these 53 underwent surgical exploration. Said another way, 20 percent of women given single-dose methotrexate will have significant pain, and 20 percent of these will require laparoscopy.
Overall, the failure rate is similar for either medical or surgical management. In three randomized trials, 5 to 14 percent of women treated initially with methotrexate ultimately required surgery, whereas 4 to 20 percent of those undergoing laparoscopic resection eventually received methotrexate for persistent trophoblast (Fernandez, 1998; Hajenius, 1997, 2007; Saraj, 1998). Rupture of persistent ectopic pregnancy is the worst form of primary therapy failure and occurs in 5 to 10 percent of women treated medically. Lipscomb and associates (1998) described a 14-day mean time to rupture, but one woman had tubal rupture 32 days after single-dose methotrexate.
Monitoring Therapy Efficacy
Serum β-hCG levels are used to monitor response to both medical and surgical therapy. After linear salpingostomy, serum β-hCG levels decline rapidly over days and then more gradually, with a mean resolution time of approximately 20 days. In contrast, after single-dose methotrexate, mean serum β-hCG levels increase for the first 4 days, and then gradually decline, with a mean resolution time of 27 days. Lipscomb and colleagues (1998) used single-dose methotrexate to successfully treat 287 women and reported that the average time to resolution—defined as a serum β-hCG level < 15 mIU/mL, was 34 days. Importantly, the longest time was 109 days.
As shown in Table 19-2, monitoring single-dose therapy calls for serum β-hCG determinations at days 4 and 7 following initial injection on day 1. If the level fails to drop more than 15 percent between days 4 and 7, then a second dose of methotrexate is required. This is necessary in 15 to 20 percent of women treated with single-dose therapy. With multidose methotrexate, levels are measured at 48-hour intervals until they fall more than 15 percent. Once appropriately dropping levels are achieved in either regimen, serum β-hCG determinations are then measured weekly until undetectable. Outpatient surveillance is preferred, but if there is any question of safety or compliance, the woman is hospitalized. Failure is judged when the β-hCG level plateaus or rises or tubal rupture occurs. Importantly, tubal rupture can occur in the face of declining β-hCG levels.
 Surgical Management
Surgical Management
Laparoscopy is the preferred surgical treatment for ectopic pregnancy unless a woman is hemodynamically unstable. There have been only a few prospective studies that compare laparotomy with laparoscopic surgery. Hajenius and associates (2007) performed a Cochrane Database review and found that subsequent uterine pregnancy rates and tubal patency rates in those treated with salpingostomy were comparable with either abdominal entry route. Subsequent ectopic pregnancies were fewer in women treated laparoscopically, although this was not statistically significant. As experience has accrued, cases previously managed by laparotomy—for example, ruptured tubal pregnancies or interstitial pregnancies—can safely be managed laparoscopically by those with suitable expertise. Before surgery, future fertility desires of the patient should be discussed. In those desiring permanent sterilization, the unaffected tube can be ligated concurrently with salpingectomy for the affected fallopian tube.
Tubal surgery is considered conservative when there is tubal salvage, such as with salpingostomy. Radical surgery is defined by salpingectomy. Some have shown conservative surgery may increase the rate of subsequent uterine pregnancy but is associated with higher rates of persistently functioning trophoblast (Bangsgaard, 2003; de Bennetot, 2012). This may not be the case, however. In a randomized controlled trial, Fernandez and associates (2013) evaluated 2-year rates of attaining an intrauterine pregnancy following either salpingostomy or salpingectomy. Although pregnancy rates with salpingectomy were 64 percent compared with 71 percent following salpingostomy, these differences were not statistically significant.
Salpingostomy
This procedure is typically used to remove a small unruptured pregnancy that is usually < 2 cm in length and located in the distal third of the fallopian tube (Fig. 19-8). Natale and associates (2003) reported that serum β-hCG levels > 6000 mIU/mL are associated with a higher risk of implantation into the muscularis and thus with more tubal damage.
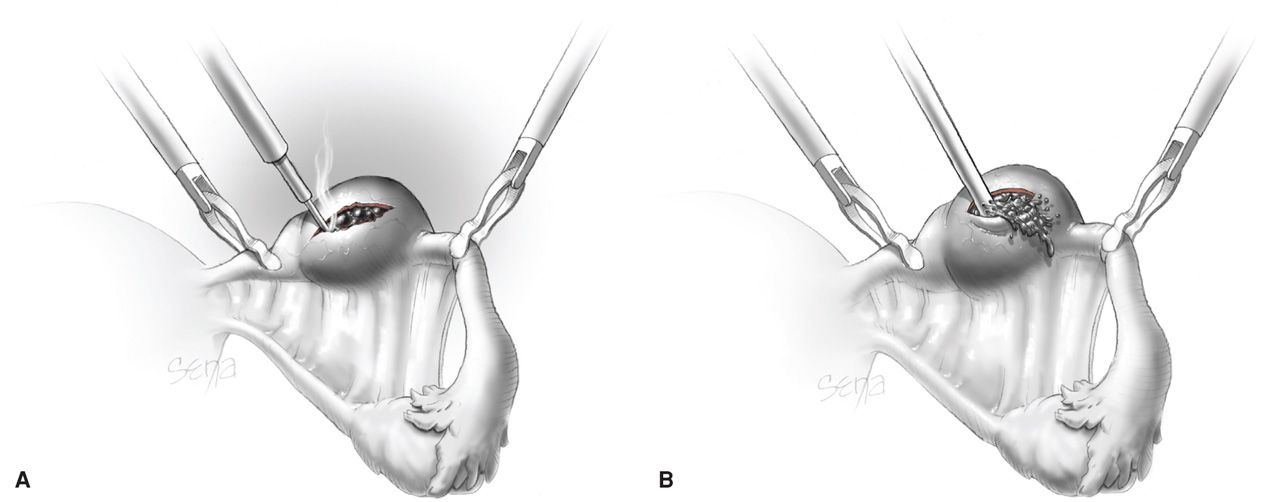
FIGURE 19-8 Linear salpingostomy for ectopic pregnancy. A. A linear incision for removal of a small tubal pregnancy is created on the antimesenteric border of the tube. B. Products of conception may be flushed from the tube using an irrigation probe. Alternatively, products may be removed with grasping forceps. Following evacuation of the tube, bleeding sites are treated with electrosurgical coagulation. The incision is not sutured. (From Thompson, 2012, with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


