Chapter 62 Early-Onset Group B Streptococcal Disease (Case 25)
Case
Speaking Intelligently
Up to 30% of women of childbearing age have rectovaginal colonization with GBS, and there is a 50% rate of vertical transmission from a colonized mother to the fetus. Up to 2% of colonized neonates develop early-onset group B streptococcus (EOGBS) disease.1–3 Before the adoption of protocols, there were up to 7500 cases annually in the United States. The 1996 Centers for Disease Control and Prevention (CDC) guideline for prevention of early-onset group B streptococcal disease specified identification of risk factors for invasive GBS disease, and specified administration of intrapartum antibiotic prophylaxis (IAP) during labor to those mothers with either a positive GBS culture or specific risk factors.4 Since the 2002 CDC guideline recommended universal screening of pregnant women at 35 to 37 weeks gestation with administration of IAP to all who test positive, there has been a decline of over 70% in EOGBS disease. Despite the marked decline in incidence, GBS remains the chief cause of neonatal infectious disease. The 2010 revised CDC guideline maintains universal antenatal screening at 35 to 37 weeks gestation with IAP for GBS-positive mothers. In addition, women with an unknown GBS status and one or more risk factors: gestation less than 37 weeks, prolonged rupture of membranes (PROM), temperature of at least 100.4° F, GBS bacteruria at any time during the pregnancy, and history of a previous infant with invasive GBS disease regardless of current maternal GBS status should continue to receive IAP. The new guideline introduces use of point-of-care nucleic acid amplification testing (NAAT), and clarifies criteria for prophylaxis in penicillin allergic mothers.1,5
Patient Care
History
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


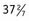 -week infant with Apgar scores 8 and 9 presents at 1 hour of life with hypoxia, hypothermia, and tachypnea. Prenatal laboratory test results were normal except for unknown maternal group B streptococcus status. On examination, the infant is in moderate respiratory distress with tachypnea, flaring, retractions, grunting, and crackles at lung bases. There is a grade II/VI systolic murmur at the left midsternal border. Oxygen saturation is 88% on room air.
-week infant with Apgar scores 8 and 9 presents at 1 hour of life with hypoxia, hypothermia, and tachypnea. Prenatal laboratory test results were normal except for unknown maternal group B streptococcus status. On examination, the infant is in moderate respiratory distress with tachypnea, flaring, retractions, grunting, and crackles at lung bases. There is a grade II/VI systolic murmur at the left midsternal border. Oxygen saturation is 88% on room air.