Fig. 3.1
Current model of osteoblast differentiation from stem cell. Ihh is the initiator of endochondral ossification. The Runx2-expressing bipotential progenitors can differentiate into either osteoblast or chondrocyte. Then cells differentiate into preosteoblasts, in which Runx2 plays an essential role. In the next step, preosteoblasts differentiate into mature osteoblasts, a process in which Osx plays a critical role. Osx is an osteoblast-specific transcription factor required for osteoblast differentiation and bone formation
Osx Is Required and Specific for Bone Formation
Osx is the only bone-specific transcription factor identified so far which is required for bone formation. Osx knockout is lethal. Heterozygous Osx mutant mice were normal and fertile. Homozygous Osx mutant mice had difficulty in breathing, and died within 15 min of birth [18]. Osteoblast marker genes such as ALP and OC are undetectable in Osx-null mutant. There is no bone formation without Osx. On the other hand, it was shown that Osx was sufficient to induce the expression of osteoblast marker OC in mesenchymal stem cells in vitro [18]. Osx controls osteogenesis as a downstream gene of Runx2 [18]. Runx2 is required for bone formation; however, Runx2 is expressed in different cells and tissues, including osteoblasts, chondrocytes, epithelial cells, glioma cells, brain tissues, and different tumor tissues [23]. Unlike Runx2, Osx is unique and bone-specific in that it is specifically expressed in osteoblasts and at low levels in prehypertrophic chondrocytes [18]. Osx is not only critical for embryonic bone formation but also essential in postnatal bone growth and in bone homeostasis using the conditional knockout approach [24].
Despite the discovery of its significance in skeletal physiology a decade ago [18], relatively little is known about direct target genes for Osx and molecular mechanisms through which Osx controls gene transcription. Recently, our research laboratory has identified several downstream bone-related target genes of Osx during bone formation, including Satb2, VDR, VEGF, SOST, DKK1, and MMP13 [25–30]. Identification of VEGF as a downstream direct target of Osx also indicates that Osx plays an important role in coordinating osteogenesis and angiogenesis [27]. These Osx downstream targets each play important roles during bone formation (Fig. 3.2), supporting the notion that Osx, as a bone-specific transcription factor, is a master gene for osteoblast differentiation and bone formation.
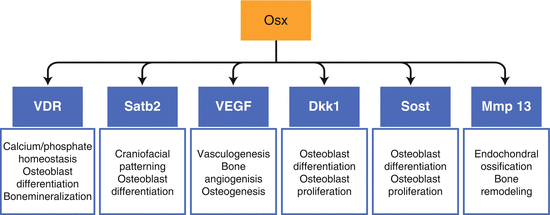

Fig. 3.2
Osx downstream target genes in osteoblasts. Downstream bone-related target genes of Osx during bone formation have been identified in our research laboratory, including Satb2, VDR, VEGF, SOST, DKK1, and MMP13. These Osx downstream targets play different important roles during bone formation, supporting the notion that Osx as an osteoblast-specific transcription factor is a master gene for osteoblast differentiation and bone formation
Identification of Potential Chemical Leads from HTS
Identification of anabolic agents that can stimulate bone formation to treat osteoporosis has been recognized as a priority in the bone biology field. Recent genome-wide association studies have shown that Osx are associated with bone mineral density in both children and adults, suggesting that Osx may contribute to the cause of osteoporosis [31, 32]. Because of the tissue specificity and the critical role of Osx in bone formation, Osx can be considered an ideal novel target for the development of a therapeutic strategy to induce the anabolic pathway of bone synthesis. At present, no pharmacological approach to target Osx in osteoblasts has been identified. Focusing on novel target Osx that are responsible for driving osteoblast differentiation and bone formation increases the likelihood of discovering mechanism-based agents that are more effective and less toxic than drugs of the previous era. Therefore, HTS assay must be performed to identify compounds promoting osteoblast differentiation and bone formation through Osx. In this case, this mechanism-based approach aims to identify potential anabolic agents by targeting a bone-specific factor Osx. Protocols for assay development and HTS execution are shown in Fig. 3.3. Parameters and controls (positive, and neutral) for the proposed primary assay must be developed, refined, and validated such that it is robust (Z′ values ≥ 0.45 over many assays and experimental days) [33], tolerant of effects from DMSO, free from systematic effects (e.g., plating artifacts, liquid handling errors), simple (most assays have less than three liquid additions and are endpoint assays), and efficient in use of reagents and resources. Secondary assays will be developed in parallel to the primary assay and meet similar criteria. Candidate small molecules identified by the process will be validated and characterized for their osteogenic activities using in vitro assays. The role of candidate small molecules in osteogenesis in vivo will be explored in osteoporosis animal models.
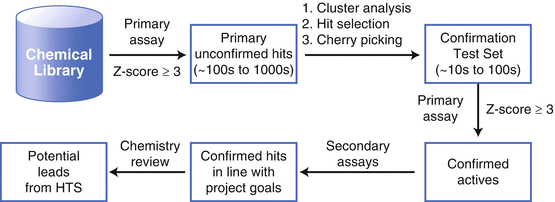

Fig. 3.3
Identification of chemical leads from high throughput screening. The following protocol will be used for assay development and HTS execution. Secondary assays will be developed in parallel to the primary assay and meet similar criteria
Overview of Drug Development Approval Process in the USA
How Drugs Are Developed and Approved by FDA
The FDA is an agency of the federal government’s Department of Health and Human Services. Center for Drug Evaluation and Research (CDER) is the largest of the FDA’s five centers in the USA. CDER is in charge of both prescription and nonprescription or over-the-counter drugs. The CDER mission is to ensure that drugs marketed in the USA are safe and effective. CDER does not test drugs, although the Center’s Office of Testing and Research does conduct limited research in the areas of drug quality, safety, and effectiveness. CDER activities include (1) reviewing drugs before marketing, (2) watching for drug problems, (3) monitoring drug information and advertising, (4) scientific research, and (5) protecting drug quality.
Companies must apply to the FDA in order to introduce a new drug into the US market. Companies have the responsibility to test the drug and submit evidence that the drug is safe and effective. A team of CDER physicians, statisticians, chemists, pharmacologists, and other scientists reviews new drug applications.
Pediatric Drug Development
The FDA Amendments Act of 2007 reauthorizes and amends the Best Pharmaceuticals for Children Act of 2002 (BPCA) and the Pediatric Research Equity Act of 2003 (PREA), both of which encourage more research in pediatric drug development. Some notable changes to BPCA and PREA are (1) authorization to establish an internal review committee (the Pediatric Review Committee will review requests for waivers and deferrals, pediatric assessments and pediatric plans prior to approval, and pediatric written requests prior to issuance); (2) clinical, clinical pharmacology, and statistical reviews are to be made public for applications submitted in response to both PREA and BPCA; and (3) adverse event reporting now affects both PREA and BPCA (review of reports has been modified to occur 1 year after labeling approval).
Introduction of Best Pharmaceuticals for Children Act
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) needs to oversee the activities of BPCA. The BPCA program aims to improve pediatric therapeutics through preclinical and clinical drug trials that result in drug labeling changes. Federal legislation and FDA regulations require that drugs be tested for safety and efficacy in a specific population, at a specific dosage, and for a specific time period before the drugs are finally approved for clinical use. Use of drugs without appropriate testing is considered “off-label” use.
Testing drugs in children comes with scientific, clinical, ethical, technical, and logistical challenges. Several practical challenges have discouraged drug testing in pediatric populations. These challenges include (1) lack of incentives for companies to conduct research on drugs in neonates, infants, and children; (2) lack of necessary technology to monitor patients and assay very small amounts of blood; and (3) lack of suitable infrastructure for conducting pediatric pharmacology drug trials. As a result, the majority of drugs used in children are used off-label, without adequate understanding of appropriate dose, safety, or efficacy. This encourages pharmaceutical companies to conduct pediatric studies of on-patent drugs that are used in pediatric populations, but are not labeled for such use, by extending their market exclusivity.
BPCA Prioritization Process
The Eunice Kennedy Shriver NICHD has sought public input and obtained different collaboration within National Institutes of Health with experts in pediatrics to identify drugs in need of further study and to prioritize needs in pediatric therapeutics. Following the 2007 legislation changes, the procedure for prioritization was revised to emphasize knowledge gaps in therapeutic areas as opposed to those about specific drug products. Specifically, the legislation authorizes that the NIH, in consultation with the Commissioner of Food and Drugs as well as researchers with expertise in pediatric research, shall develop and publish a priority list of needs in pediatric therapeutics, including drugs or indications that require study. This list shall be revised every 3 years. The revised legislation also required that, in developing these priorities, the Secretary shall consider (1) therapeutic gaps in pediatrics that may include developmental pharmacology, pharmacogenetic determinants of drug response, metabolism of drugs and biologics in children, and pediatric clinical trials; (2) particular pediatric diseases, disorders, or conditions where more complex knowledge and testing of therapeutics, including drugs and biologics, may be beneficial in pediatric populations; and (3) the adequacy of necessary infrastructure to conduct pediatric pharmacological research, including research networks and trained pediatric investigators.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


