- Low birthweight (LBW): <2500 g (7% of UK births)
- Very low birthweight (VLBW): <1500 g (1% of UK births)
- Extremely low birthweight: <1000 g
- Preterm: <37 weeks gestation (6% of UK births)
- Term: 37–41 weeks gestation
- Post-term: ≥42 weeks gestation
- Small for gestational age (SGA): birthweight <2nd centile for gestation
- Large for gestational age: birthweight >98th centile for gestation
- Appropriate for gestational age (AGA): birthweight 2nd to 98th centile for gestation.
 Different problems occur when low birth weight is due to prematurity or intrauterine growth restriction.
Different problems occur when low birth weight is due to prematurity or intrauterine growth restriction.The best guide to gestation is the menstrual history and ultrasound estimate of fetal size made before 20 weeks’ gestation. Physical and neurological features of the newborn change with gestation. As gestation increases, skin becomes keratinized, thicker, and has less lanugo (fine hair). Ear cartilage and the breast nodule develop during the third trimester. In boys, the testes descend into the scrotum at around 36 weeks. In girls, the labia majora cover the labia minora towards term. As gestation increases, muscle tone and ligamentous laxity increase.
8.2 The preterm baby
- Respiratory distress syndrome (surfactant deficiency)
- Recurrent apnoea
- Poor thermoregulation
- Renal function, fluid and electrolyte balance
- Nutrition
- Patent ductus arteriosus
- Intraventricular haemorrhage and other brain damage
- Anaemia
- Necrotizing enterocolitis
- Jaundice.
8.2.1 Respiratory distress syndrome (RDS)
RDS, also known as hyaline membrane disease, is virtually confined to preterm babies and is common below 32 weeks.
 PRACTICE POINT
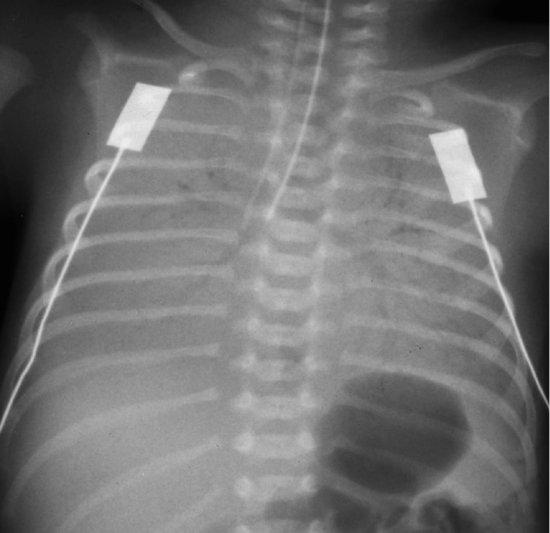
PRACTICE POINTSurfactant lowers surface tension in the liquid that lines the respiratory tract. Without surfactant, the alveoli cannot be inflated and tend to collapse. The result is atelectasis (poor aeration) and the appearance on X-ray (Figure 8.2). Increased work of breathing (respiratory distress) occurs, leading to poor oxygenation and carbon dioxide retention (respiratory failure), and often to apnoeic spells.
Figure 8.2 Respiratory distress syndrome: note the characteristic ground glass appearance in all lung fields. In this picture, the tracheal tube is too far down, and was pulled back afterwards.
 Respiratory distress
Respiratory distress- Tachypnoea (>60 bpm)
- Intercostal recession
- Subcostal recession
- Grunting
- Nasal flare
- Cyanosis.
Surfactant replacement therapy can be given through the endotracheal tube soon after birth, and leads to reduced mortality, less risk of pneumothorax and reduced lung damage. RDS resolves over a period of 3–7 days as surfactant production increases. The key to management is to keep the baby alive and undamaged during this period. Infants with significant RDS need assisted ventilation (mechanical ventilation or continuous positive airway pressure). Careful monitoring is crucial.
8.2.2 Bronchopulmonary dysplasia (BPD)
This chronic lung disease occurs in some preterm babies who have been ventilated for severe RDS. It is due to lung damage by high oxygen concentration and high positive pressures. Steroid therapy is helpful in ventilator-dependent infants, but has side effects. Some babies with BPD require prolonged oxygen therapy for months.
 PRACTICE POINT Blood gas stability is essential
PRACTICE POINT Blood gas stability is essential| Blood oxygen | Blood carbon dioxide | |
| Low | Tissue or brain damage | Brain ischaemia and PVL |
| High | Retinopathy of prematurity | Intracranial haemorrhage |
8.2.3 Recurrent apnoea
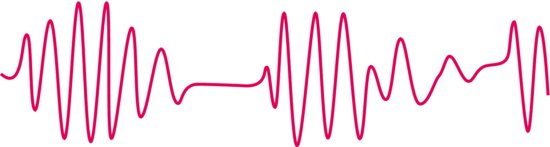
Preterm infants do not breathe regularly (Figure 8.3). If periods of shallow breathing or apnoea exceed 10–20s, they are associated with bradycardia and cyanosis. Apnoeas may be the first sign of neonatal illness but commonly are due to immaturity. Caffeine is given to stimulate regular breathing. Apnoea monitors are in regular use on neonatal units! (see Figure 28.2f).
8.2.3.1 Temperature control
Hypothermia increases oxygen consumption and predisposes to RDS and infection. It must be avoided. Incubators and radiant heaters are used so that the baby uses little energy keeping warm or cooling down.
 PRACTICE POINT
PRACTICE POINT- Large surface area relative to body mass
- Little subcutaneous fat
- Large insensible water and heat loss.
8.2.4 Fluid and electrolyte balance
Renal function is relatively poor in the preterm baby. Coupled with a high (but largely unmeasurable) water-loss through the very permeable skin, this can lead rapidly to dehydration and electrolyte disturbance.
8.2.5 Nutrition
If the preterm newborn is to equal the fetal growth rate, requirements are high. If the baby is not ill, milk is given. Frequent, small volume nasogastric feeds are used. The best milk is the mother’s expressed breast milk – it is better tolerated, induces gut maturation and reduces risk of necrotizing enterocolitis. In VLBW infants, protein and calories are added to breast milk. Nutritive sucking and swallowing develops at about 34 weeks. For tiny sick babies, parenteral nutrition is often used.
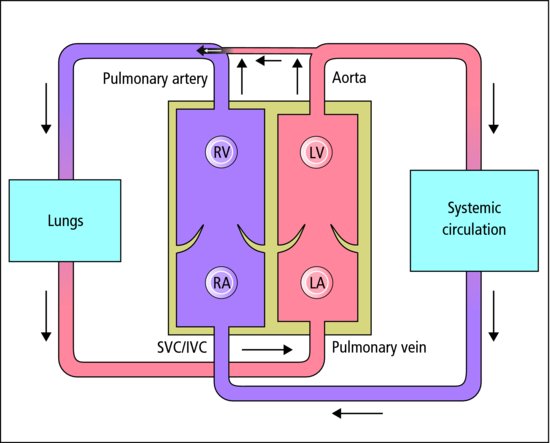
8.2.6 Patent ductus arteriosus (PDA)
The ductus arteriosus (Figure 8.4) of the preterm infant often fails to close, especially when RDS is present. Left-to-right shunting may lead to heart failure. Most close with time. The PDA can be closed by giving a prostaglandin synthetase inhibitor (e.g. ibuprofen), but sometimes surgical closure is required.
8.2.7 Intraventricular haemorrhage and other forms of brain damage
In the walls of the lateral ventricles of the preterm baby’s brain are fragile capillaries (the germinal matrix), where bleeding may occur. Haemorrhage, easily seen on cranial ultrasound through the fontanelle, may be local or extend into the ventricles or brain tissue. The more extensive the haemorrhage, the more likely are brain damage and hydrocephalus. It is estimated that some degree of haemorrhage occurs in 40% of very small babies, although it causes serious damage in only a small minority.
Periventricular leukomalacia (PVL) is the most important pattern of brain damage in the preterm. It is seen in 5–10% under 32 weeks. White matter beside the ventricle is damaged by hypoxia/ischaemia and also inflammation and low carbon dioxide levels. It characteristically leads to spastic diplegia.
8.2.8 Anaemia
Anaemia is common because of red cell breakdown, and ‘bleeding into the laboratory’. Transfusion is often used. Extra iron is given with breast feeding when the preterm infant is 6 weeks old. Preterm formula milk is supplemented with iron.
8.2.9 Necrotizing enterocolitis (NEC)
NEC is the most common surgical emergency in neonatal medicine. Surgery is often needed. Mortality is 20%. The infant becomes sick and acidotic with a distended abdomen due to gut necrosis. NEC is almost restricted to the preterm. Aetiology is unknown, but immaturity, infection, gut ischaemia and enteral milk feeding all contribute to pathogenesis. Breast milk feeds reduce the risk of NEC considerably.
8.2.10 Management of the preterm infant
Preterm infants who are unwell are looked after in neonatal units or special care baby units (SCBUs) (Figure 8.5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree