103 Disorders of the Gastrointestinal System
Intestinal Obstruction
Clinical Presentation
Subtle differences in presentation can suggest the location of the obstruction along the alimentary tract (Table 103-1). These unique features are highlighted below as each disorder is discussed.
Table 103-1 Localizing Features of Intestinal Obstruction
| Sign or Finding | Proximal or Distal | Specific Site (When Applicable) |
|---|---|---|
| Nonbilious vomiting | Proximal | Proximal to the ampulla of Vater |
| Bilious vomiting | Proximal or distal | Distal to the ampulla of Vater |
| Scaphoid abdomen | Proximal | Often preduodenal |
| Distended abdomen | Distal | |
| Maternal polyhydramnios | Proximal |
Esophageal Atresia
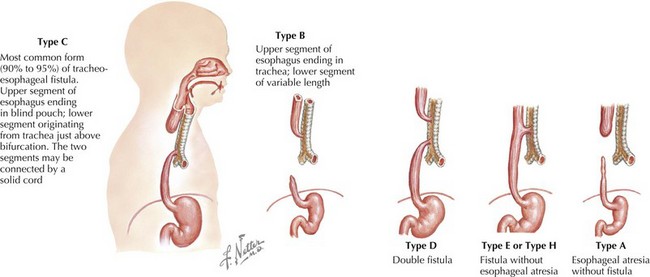
The esophagus can be obstructed in the form of esophageal atresia (EA), which is caused by a failure of separation of the esophagus from the trachea during normal development. Pure EA is rare and often coexists with tracheoesophageal fistula (TEF). A standard classification scheme describes the relationship between the atresia and the coexistent TEF (Figure 103-1). The most common combination is EA with distal TEF (type C). The most difficult to recognize and diagnose is “H-type,” which refers to isolated TEF without EA.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree