70 Disorders of the Adrenal Gland
Adrenal Gland Physiology
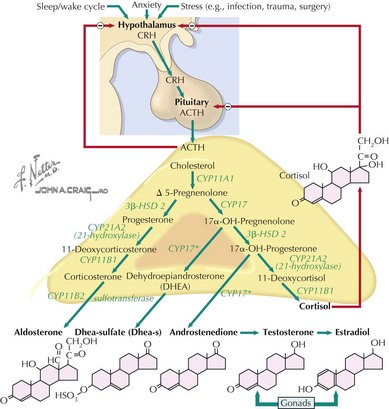
The adrenal cortex consists of three distinct zones, the glomerulosa, the fasciculata, and the reticularis. The fasciculata is the principal component of the hypothalamic–pituitary–adrenal axis. Glucocorticoid (cortisol) secretion from the fasciculata is regulated by adrenocorticotropic hormone (ACTH). ACTH is synthesized from pre-pro-opiomelanocortin (pre-POMC). The removal of the signal peptide during translation produces the 267 amino acid polypeptide POMC, which undergoes a series of posttranslational modifications to yield various polypeptide fragments with varying physiological activity. These fragments include the 39 amino acid polypeptide ACTH, as well as β-lipotropin, γ-lipotropin, melanocyte-stimulating hormone (α-MSH), and β-endorphin. POMC, ACTH and β-lipotropin are secreted from corticotropes in the anterior lobe of the pituitary gland in response to the hormone corticotropin-releasing hormone (CRH) released by the hypothalamus (Figure 70-1).
Functions of Adrenal Hormones
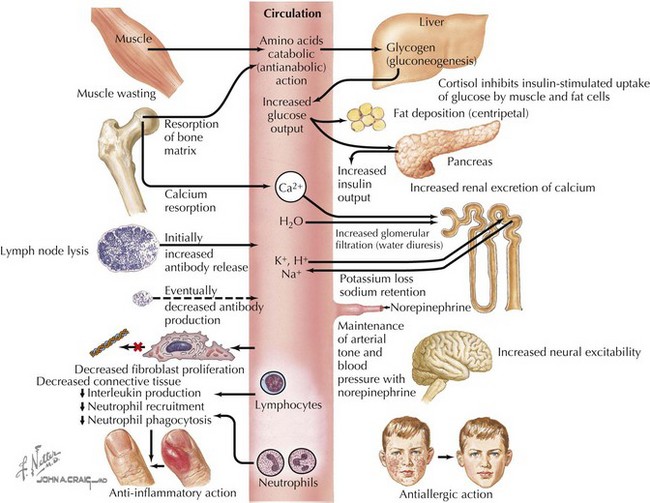
Cortisol plays important roles in cardiovascular stability (maintaining blood pressure by increasing the sensitivity of the vasculature to the vasoconstrictive effects of epinephrine and norepinephrine), metabolism (increasing gluconeogenesis to prevent hypoglycemia), and fluid and electrolyte balance (sodium retention and potassium excretion). It also inhibits bone formation and is a potent antiinflammatory agent (Figure 70-2).
Aldosterone acts on the distal tubules and collecting ducts of the kidney to increase conservation of sodium and water, secrete potassium, and increase blood pressure. Adrenal androgens produced in the zona reticularis play a role in pubarche and the development of secondary sexual characteristics (i.e., pubic hair) in both males and females during puberty (see Chapter 67).
Adrenal Insufficiency
Etiology and Pathogenesis
Primary Adrenal Insufficiency
Primary adrenal insufficiency is caused by congenital or acquired dysfunction of the adrenal cortex or the hormone-producing steroidogenic pathway (Table 70-1). The most common cause in the developed world is autoimmune adrenalitis, also known as Addison’s disease. In developing countries, tuberculosis remains the most prominent cause. Destruction of the gland leads to deficiencies in all adrenal cortex hormones, but this process may be metasynchronous, and not all hormones are lacking in all patients. Enzyme defects can cause cortisol deficiency with an excess of precursor hormones. Loss of negative feedback from low cortisol levels leads to high ACTH in these disorders.
Table 70-1 Causes of Primary Adrenal Insufficiency
| Cause | Associations or Pathogenesis | Diagnosis |
|---|---|---|
| Acquired | ||
| Addison’s disease | Other autoimmune disease | Adrenal antibodies |
| Autoimmune polyglandular syndrome | Type 1: hypoparathyroidism and mucocutaneous candidiasis Type 2: type 1 diabetes, thyroiditis, other autoimmune | AIRE gene mutation in type 1 |
| Infiltration or infection | TB, fungal, cancer, amyloidosis, sarcoid, hemochromatosis, CMV (HIV patients) | PPD, cultures, imaging, biopsy, ELISA or Western blot |
| Waterhouse-Friderichsen syndrome | Meningococcemia leading to adrenal hemorrhage | Cultures |
| Bilateral hemorrhage | Trauma, anticoagulants | Imaging |
| Medications: mitotane, ketoconazole | Destruction of gland, enzyme blockage | History |
| Congenital | ||
| CAH | Autosomal recessive; mutation of 21-hydroxylase and others | Adrenal steroid profiles, genetic testing CYP21 |
| Adrenoleukodystrophy; adrenomyeloneuropathy | X-linked; buildup of VLCFAs in adrenals and cerebral or spinal cord involvement; neuromuscular disease | Serum VLCFAs; ALD gene |
| X-linked congenital adrenal hypoplasia | Delayed puberty, contiguous gene mutations (Duchenne’s muscular dystrophy, glycerol kinase) | DAX1 gene testing |
| Triple A syndrome | Autosomal recessive; achalasia, alacrima, adrenal insufficiency | AAAS gene at 12q13 |
| Other syndromes: IMAGE, Smith-Lemli-Opitz | Vary | Genetic testing |
| ACTH resistance | ACTH receptor or melanocortin 2 receptor accessory protein (MRAP) gene mutations | Genotyping of receptor or MRAP genes |
ACTH, adrenocorticotropic hormone; CAH, congenital adrenal hyper-plasia; CMV, cytomegalovirus; ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; IMAGE, intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenita, genital abnormalities; PPD, purified protein derivative; TB, tuberculosis; VLCFA, very long chain fatty acid.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree