Chapter 83 Disorders of Purine and Pyrimidine Metabolism
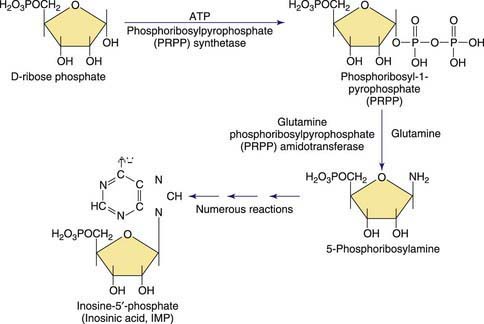
Purines are involved in all biologic processes; all cells require a balanced supply of purines for growth and survival. They provide the primary source of cellular energy through adenosine triphosphate (ATP) and, together with pyrimidines, provide the source for the RNA and DNA that stores, transcribes, and translates genetic information. Purines provide the basic coenzymes (NAD, NADH) for metabolic regulation and play a major role in signal transduction (GTP, cAMP, cGMP). Metabolically active nucleotides are formed from heterocyclic nitrogen-containing purine bases (guanine and adenine) and pyrimidine bases (cytosine, uridine, and thymine). The early steps in the biosynthesis of the purine ring are shown in Figure 83-1. Purines are primarily produced from endogenous sources and, in usual circumstances, dietary purines have a small role. The end product of purine metabolism in humans is uric acid (2,6,8-trioxypurine).
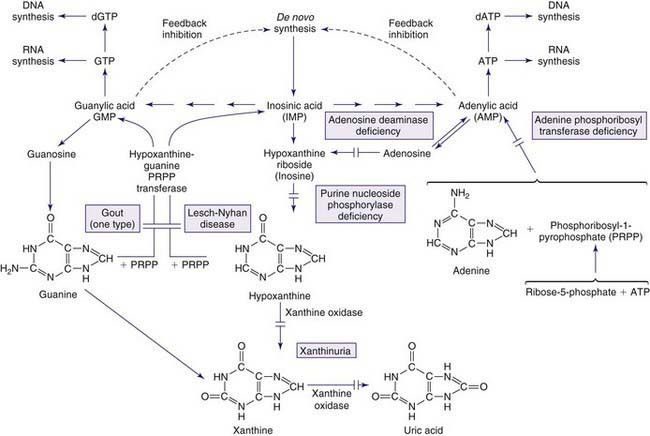
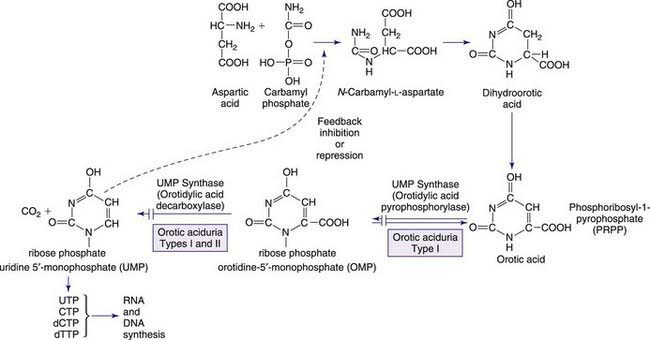
The metabolism of both purines and pyrimidines can be divided into 2 biosynthetic pathways and a catabolic pathway. The 1st, the de novo pathway, involves a multistep biosynthesis of phosphorylated ring structures from precursors such as CO2, glycine, and glutamine. Purine and pyrimidine nucleotides are produced from ribose-5-phosphate or carbamyl phosphate, respectively. The 2nd, a single-step salvage pathway, recovers purine and pyrimidine bases derived from either dietary intake or the catabolic pathway (Figs. 83-2 and 83-3; also see Fig. 83-1). In the de novo pathway, the nucleosides guanosine, adenosine, cytidine, uridine, and thymidine are formed by the addition of ribose-1-phosphate to the purine bases guanine or adenine, and to the pyrimidine bases cytosine, uracil, and thymine. The phosphorylation of these nucleosides produces monophosphate, diphosphate, and triphosphate nucleotides. Under usual circumstances, the salvage pathway predominates over the biosynthetic pathway. Synthesis is most active in tissues with high rates of cellular turnover, such as gut epithelium, skin, and bone marrow. The 3rd pathway is catabolism. The end product of the catabolic pathway of the purines is uric acid, whereas catabolism of pyrimidines produces citric acid cycle intermediates. Only a small fraction of the purines turned over each day are degraded and excreted.
Gout
Gout is associated with hereditary disorders in three different enzyme disorders that result in hyperuricemia. These include the severe form of HPRT deficiency (Lesch-Nyhan disease) and partial HPRT deficiency, superactivity of PP-ribose-P synthetase, and glycogen storage disease type I (glucose-6-phosphatase deficiency) (Chapter 81.1). In the 1st two, the basis of hyperuricemia is purine nucleotide and uric acid overproduction, whereas in the 3rd, it is both excessive uric acid production and diminished renal excretion of urate. Glycogen storage disease types III, V, and VII are associated with exercise-induced hyperuricemia, the consequence of rapid ATP utilization and failure to regenerate it effectively during exercise (Chapter 81.1). Autosomal dominant juvenile hyperuricemia, gouty arthritis, medullary cysts, and progressive renal insufficiency are features associated with familial juvenile hyperuricemic nephropathy (FJHN) and medullary cystic kidney disease type 1 (MCKD1) and type 2 (MCKD2). MCKD1 has been mapped to chromosome 1q21. FJHN and MCKD2 have been mapped to chromosome 16p11.2. FJHN and MCKD2 are proposed to be allelic and can result from uromodulin (UMOD) mutations; the term uromodulin-associated kidney disease (UAKD) has been proposed. Unlike the three inherited purine disorders that are X-linked and the recessively inherited glycogen storage disease, these are autosomal dominant conditions. Familial juvenile gout or familial juvenile hyperuricemic nephropathy is associated with severe renal hypoexcretion of uric acid. Although it most commonly presents from puberty up to the 3rd decade, it has been reported in infancy. It is characterized by early onset, hyperuricemia, gout, familial renal disease, and low urate clearance relative to glomerular filtration rate. It occurs in both males and females and is frequently associated with a rapid decline in renal function that may lead to death unless diagnosed and treated early. Once FJHN is recognized, presymptomatic detection is of critical importance to identify asymptomatic family members with hyperuricemia and to begin treatment, when indicated, to prevent nephropathy.
Abnormalities in Purine Salvage
Lesch-Nyhan Disease (LND)
Clinical Manifestations
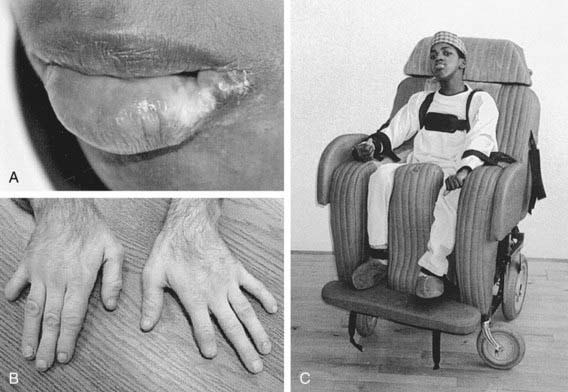
The age of onset of self-injury may be as early as 1 yr and, occasionally, as late as the teens. Self-injury occurs, although all sensory modalities, including pain, are intact. The self-injurious behavior usually begins with self-biting, although other patterns of self-injurious behavior emerge with time. Most characteristically, the fingers, mouth, and buccal mucosa are mutilated. Self-biting is intense and causes tissue damage and may result in the amputation of fingers and substantial loss of tissue around the lips (Fig. 83-4). Extraction of primary teeth may be required. The biting pattern can be asymmetric, with preferential mutilation of the left or right side of the body. The type of behavior is different from that seen in other intellectual disability syndromes involving self-injury; self-hitting and head banging are the most common initial presentations in other syndromes. The intensity of the self-injurious behavior generally requires that the patient be restrained. When restraints are removed, the individual with LND may appear terrified, and stereotypically place a finger in the mouth. The patient may ask for restraints to prevent elbow movement; when the restraints are placed or replaced, the patient may appear relaxed and better humored. Dysarthric speech may cause interpersonal communication problems; the higher-functioning children can express themselves fully and participate in verbal therapy.